��Ŀ����
��19�֣�N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ��______________��
��2��һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��2N2O5(g) 4NO2(g)��O2(g)����H��0
4NO2(g)��O2(g)����H��0
�ٷ�Ӧ�ﵽƽ�������ͨ��һ������������N2O5��ת���ʽ�______�����������С���������䡱����
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
��500 s��N2O5�ķֽ�����Ϊ________________��
����T2�¶��£���Ӧ1000 sʱ���NO2��Ũ��Ϊ4.98 mol��L��1����T2________T1������>��<��
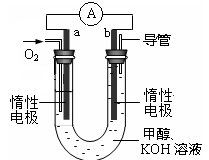
��3������ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�______�����ɣ���缫��ӦʽΪ_______��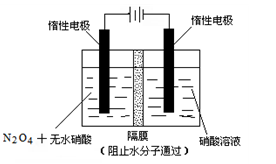
��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ��______________��
��2��һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��2N2O5(g)
 4NO2(g)��O2(g)����H��0
4NO2(g)��O2(g)����H��0�ٷ�Ӧ�ﵽƽ�������ͨ��һ������������N2O5��ת���ʽ�______�����������С���������䡱����
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
| t/s | 0 | 500 | 1000 |
| c(N2O5)/mol��L��1 | 5.00 | 3.52 | 2.48 |
����T2�¶��£���Ӧ1000 sʱ���NO2��Ũ��Ϊ4.98 mol��L��1����T2________T1������>��<��
��3������ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�______�����ɣ���缫��ӦʽΪ_______��

��1��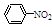 ����3�֣���2���ٲ��䣨3�֣� ��0.00296 mol��L��1��s��1��3�֣�
����3�֣���2���ٲ��䣨3�֣� ��0.00296 mol��L��1��s��1��3�֣�
�ۣ���3�֣���3��������3�֣� N2O4��2HNO3��2e����2N2O5��2H�� ��4�֣�
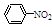 ����3�֣���2���ٲ��䣨3�֣� ��0.00296 mol��L��1��s��1��3�֣�
����3�֣���2���ٲ��䣨3�֣� ��0.00296 mol��L��1��s��1��3�֣��ۣ���3�֣���3��������3�֣� N2O4��2HNO3��2e����2N2O5��2H�� ��4�֣�
��1���������л��У�NO2�����ţ��ṹ��ʽΪ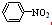 ��
��
��2����ͨ�뵪���������ʵ�Ũ���Dz���ģ�����ƽ�ⲻ�ƶ���ת���ʲ��䡣
��500sʱ������N2O5��Ũ����1.48mol/L�����Էֽ�������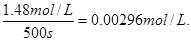
��NO2��4.98mol/L��������N2O5��2.49mol/L��ʣ��N2O5��2.51mol/L�����¶�ΪT1ʱ��2.48mol/L����˵���¶�ΪT1ʱ��Ӧ���ʿ죬����T1����T2��
��3��N2O5�е�Ԫ�صĻ��ϼ��ǣ�5�ۣ��������е�Ԫ��Ҳ�ǣ�5�ۡ����Ӧ�����������N2O5�����������������ɣ�����ʽΪ N2O4��2HNO3��2e����2N2O5��2H����
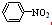 ��
����2����ͨ�뵪���������ʵ�Ũ���Dz���ģ�����ƽ�ⲻ�ƶ���ת���ʲ��䡣
��500sʱ������N2O5��Ũ����1.48mol/L�����Էֽ�������
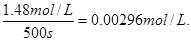
��NO2��4.98mol/L��������N2O5��2.49mol/L��ʣ��N2O5��2.51mol/L�����¶�ΪT1ʱ��2.48mol/L����˵���¶�ΪT1ʱ��Ӧ���ʿ죬����T1����T2��
��3��N2O5�е�Ԫ�صĻ��ϼ��ǣ�5�ۣ��������е�Ԫ��Ҳ�ǣ�5�ۡ����Ӧ�����������N2O5�����������������ɣ�����ʽΪ N2O4��2HNO3��2e����2N2O5��2H����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
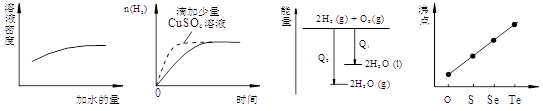
 ��ԭ�ϡ���֪��
��ԭ�ϡ���֪��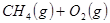
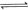



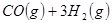




 ��
�� ��Ӧ����
��Ӧ����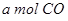 ��
�� ���ڲ�ͬѹǿ�ºϳɼ״���
���ڲ�ͬѹǿ�ºϳɼ״��� ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��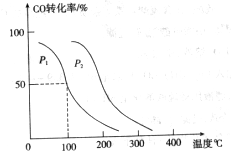

 ���<������>����=����
���<������>����=���� ��
��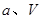 �Ĵ���ʽ��ʾ����
�Ĵ���ʽ��ʾ���� �缫ͨ���
�缫ͨ��� Ϊ ���缫��Ӧʽ�� ��
Ϊ ���缫��Ӧʽ�� ��
 ��Һ�����õ�
��Һ�����õ� ͭʱ���μӷ�Ӧ������
ͭʱ���μӷ�Ӧ������ �����ӦΪ
�����ӦΪ  ����״������
����״������ Si3N4��s��+6CO��g��
Si3N4��s��+6CO��g��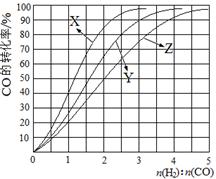
 H2(g)+CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���Իش��������⣺
H2(g)+CO2(g) ��H��0��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=1���Իش��������⣺