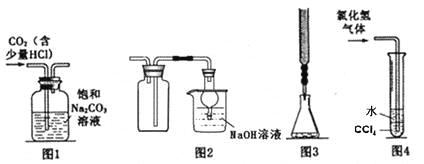
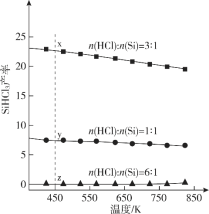
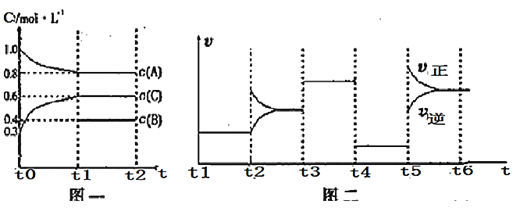
��Ŀ����
����Ŀ����֪��Ӧ2CH3OH(g) ![]() CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/mol��L��1 | 0.44 | 0.6 | 0.6 |
������������ȷ����(����)
A.�÷�Ӧ��ƽ�ⳣ������ʽΪK��![]()
B.ƽ��ʱc(CH3OH)��0.04 mol��L��1
C.��ʱ�������淴Ӧ���ʵĴ�С��v����v��
D.������CH3OH��10 min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v(CH3OH)��1.6 mol��L��1��min��1
���𰸡�B
��������
A.2CH3OH(g) ![]() CH3OCH3(g)��H2O(g)��ƽ�ⳣ������ʽK=
CH3OCH3(g)��H2O(g)��ƽ�ⳣ������ʽK=![]() ����A����
����A����
B.�ɱ������ݿ�֪���״�����ʼŨ��Ϊ0.44mol/L+2��0.6mol/L=1.64mol/L����ƽ���c(CH3OCH3)=xmol/L�����ݷ���ʽ��֪ƽ��ʱc(CH3OH)=(1.642x)mol/L��c(H2O)=xmol/L����![]() =400�����x=0.8����10min�ﵽƽ�⣬��ʱc(CH3OH)=0.04mol/L����B��ȷ��
=400�����x=0.8����10min�ﵽƽ�⣬��ʱc(CH3OH)=0.04mol/L����B��ȷ��
C. Ũ����Qc=![]() =1.34<K=400����Ӧ������Ӧ���У���ʱ�����淴Ӧ���ʵĴ�С��v��>v������C����
=1.34<K=400����Ӧ������Ӧ���У���ʱ�����淴Ӧ���ʵĴ�С��v��>v������C����
D.10min�ﵽƽ��ʱ����Ӧ����v(CH3OH)=![]() =0.16mol/(Lmin)����D����
=0.16mol/(Lmin)����D����
��ѡB��

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�