��Ŀ����
̼����(SiC) ��������(Al2O3) �͵����裨Si3N4���������ĸ��½ṹ�մɣ��ڹ�ҵ�����ͿƼ���������Ҫ��;��
��1��Al��ԭ�ӽṹʾ��ͼΪ ��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ
��
��2�������迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ��䷴Ӧ����ʽΪ ��
��3����ҵ���û�ѧ����������Ʊ������裬�䷴Ӧ���£�
3SiCl4(g) + 2N2(g) + 6H2(g) Si3N4(s) + 12HCl(g) ��H��0
Si3N4(s) + 12HCl(g) ��H��0
ij�¶Ⱥ�ѹǿ�����£��ֱ�0.3mol SiCl4(g)��0.2mol N2(g)��0.6mol H2(g)����2L�ܱ������ڣ�����������Ӧ��5min�ﵽƽ��״̬������Si3N4(s)��������5.60g��
��H2��ƽ����Ӧ������ mol��(L��min)��
��ƽ��ʱ������N2��Ũ���� mol��L-1��
��SiCl4(g)��ת������ ��
������n(SiCl4) : n(N2) : n(H2) =" 3" : 2 : 6��Ͷ����ȣ���������������������ϣ�SiCl4(g)��ת����Ӧ ����������������䡱����
�ݹ�ҵ���Ʊ����跴Ӧ���Ȼ�ѧ����ʽ���£�
SiCl4(g)��2H2(g) Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)
Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)
ij�¶ȡ�ѹǿ�£���һ�����ķ�Ӧ��ͨ���ܱ������������ϵķ�Ӧ(��������Ϊ���淴Ӧ)������������ȷ����( )
A����Ӧ�����У�������ѹǿ�����SiCl4��ת����
B������Ӧ��ʼʱSiCl4Ϊ1mol����ﵽƽ��ʱ����������ΪQkJ
C������Ӧ��������Ϊ0.025QkJʱ�����ɵ�HClͨ��100mL1mol��L��1��NaOHǡ�÷�Ӧ
D����Ӧ��4minʱ����HCl��Ũ��Ϊ0.12mol��L��1����H2�ķ�Ӧ����Ϊ0.03mol/(L��min)
��1��Al��ԭ�ӽṹʾ��ͼΪ ��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ
��
��2�������迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ��䷴Ӧ����ʽΪ ��
��3����ҵ���û�ѧ����������Ʊ������裬�䷴Ӧ���£�
3SiCl4(g) + 2N2(g) + 6H2(g)
 Si3N4(s) + 12HCl(g) ��H��0
Si3N4(s) + 12HCl(g) ��H��0 ij�¶Ⱥ�ѹǿ�����£��ֱ�0.3mol SiCl4(g)��0.2mol N2(g)��0.6mol H2(g)����2L�ܱ������ڣ�����������Ӧ��5min�ﵽƽ��״̬������Si3N4(s)��������5.60g��
��H2��ƽ����Ӧ������ mol��(L��min)��
��ƽ��ʱ������N2��Ũ���� mol��L-1��
��SiCl4(g)��ת������ ��
������n(SiCl4) : n(N2) : n(H2) =" 3" : 2 : 6��Ͷ����ȣ���������������������ϣ�SiCl4(g)��ת����Ӧ ����������������䡱����
�ݹ�ҵ���Ʊ����跴Ӧ���Ȼ�ѧ����ʽ���£�
SiCl4(g)��2H2(g)
 Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)
Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)ij�¶ȡ�ѹǿ�£���һ�����ķ�Ӧ��ͨ���ܱ������������ϵķ�Ӧ(��������Ϊ���淴Ӧ)������������ȷ����( )
A����Ӧ�����У�������ѹǿ�����SiCl4��ת����
B������Ӧ��ʼʱSiCl4Ϊ1mol����ﵽƽ��ʱ����������ΪQkJ
C������Ӧ��������Ϊ0.025QkJʱ�����ɵ�HClͨ��100mL1mol��L��1��NaOHǡ�÷�Ӧ
D����Ӧ��4minʱ����HCl��Ũ��Ϊ0.12mol��L��1����H2�ķ�Ӧ����Ϊ0.03mol/(L��min)
��1��
2Al +2OH-+2H2O = 2AlO2 + 3H2��
��2��Si3N4 +16HF = 3SiF4 + 4NH4F
��3����0.024 ��0.06 ��40% �ܲ��� �� C

2Al +2OH-+2H2O = 2AlO2 + 3H2��
��2��Si3N4 +16HF = 3SiF4 + 4NH4F
��3����0.024 ��0.06 ��40% �ܲ��� �� C
�����������1��Al��ԭ�ӽṹʾ��ͼΪ
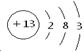 ��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al +2OH-+2H2O = 2AlO2 + 3H2����
��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al +2OH-+2H2O = 2AlO2 + 3H2������2��������������ᷴӦ�����ķ������һ����Σ����������NH4F���÷�Ӧ�Ļ�ѧ����ʽΪSi3N4 +16HF = 3SiF4 + 4NH4F ��
��3����Si3N4(s)��������5.60g�����Ե����ʵ���n=5.60/80=0.04mol������
3SiCl4(g) + 2N2(g) + 6H2(g)
 Si3N4(s) + 12HCl(g)����֪
Si3N4(s) + 12HCl(g)����֪V��H2��="6" V��Si3N4��=6����0.04/2/5��="0.024" mol��(L��min)���ڷ�Ӧ��N2�����ʵ���n=0.04��2=0.08mol������ƽ��ʱ������N2��Ũ��=��0.2-0.08��/2="0.06" mol��L-1��
��SiCl4(g)��ת������=0.04��3/0.3��100%=40%��������n(SiCl4) : n(N2) : n(H2) =" 3" : 2 : 6��Ͷ����ȣ���������������������ϣ���Ϊ���Ͷ�ϱ�Ϊ��ѧ������֮�ȣ�����SiCl4(g)��ת����Ӧ�ò��䣻����SiCl4(g)��2H2(g)
 Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)����֪����ѹǿ����Ӧ������У����Լ�Сѹǿ�����SiCl4��ת���ʣ���A��������Ӧ��ʼʱSiCl4Ϊ1mol���ﵽƽ��ʱ�����ڸ÷�ӦΪ���淴Ӧ��������������С��QkJ����B����C��ȷ����Ӧ��4minʱ����HCl��Ũ��Ϊ0.12mol��L��1����H2�ķ�Ӧ����Ϊ0.015mol/(L��min)����DҲ�������ΪC��
Si(s)��4HCl(g)����H����QkJ��mol��1(Q��0)����֪����ѹǿ����Ӧ������У����Լ�Сѹǿ�����SiCl4��ת���ʣ���A��������Ӧ��ʼʱSiCl4Ϊ1mol���ﵽƽ��ʱ�����ڸ÷�ӦΪ���淴Ӧ��������������С��QkJ����B����C��ȷ����Ӧ��4minʱ����HCl��Ũ��Ϊ0.12mol��L��1����H2�ķ�Ӧ����Ϊ0.015mol/(L��min)����DҲ�������ΪC�������������ۺϿ�����ԭ�ӽṹʾ��ͼ�����ӷ���ʽ����ѧƽ����ۺ�֪ʶ���Ǹ߿�������ص㣬����Ƚ�ȫ�棬��һ����������Ŀ��

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
 Si �� 2CO�� ��֪�ǽ����ԣ�C > Si
Si �� 2CO�� ��֪�ǽ����ԣ�C > Si