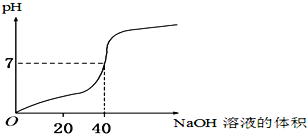
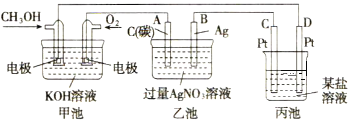
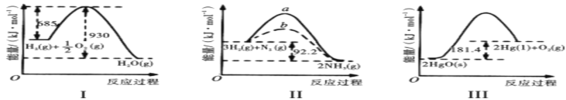
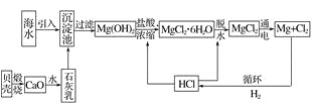
��Ŀ����
����Ŀ����ѧ������������������ء������й����ʵ���;�����ʶ���ȷ��������Ե���
ѡ�� | ��; | ���� |
A | Һ��������� | NH3�ֽ�����N2��H2�ķ�Ӧ�����ȷ�Ӧ |
B | NH4Fe��SO4��2��12H2O������ˮ�� | NH4Fe��SO4��2��12H2O���������� |
C | Ư�۾����������������� | Ư�۾���Һ��ClO-��HClO����ǿ������ |
D | Al2O3�������²��� | Al2O3��������ǿ�ᷴӦ��������ǿ�Ӧ |
A.AB.BC.CD.D
���𰸡�C
��������
A��Һ������ʱ��Ҫ�������ȣ�Һ����������������백���ķֽ��أ���A����
B��������ˮ�����������������壬ʹ��![]() ���������ԣ�������ˮ�������������أ���B����
���������ԣ�������ˮ�������������أ���B����
C��Ư�۾���Ϊ����������ΪClO��HClO����ǿ�����ԣ���C��ȷ��
D����������Ϊ���²�������Ϊ���������۵�ܸߣ����仯ѧ�����أ���D����
��ѡC��

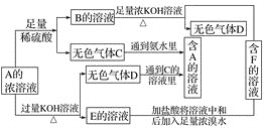
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�����Ŀ���û���̿��ԭ������������йط�ӦΪC(s)+2NO(g) ![]() N2(g)+CO2(g)��
N2(g)+CO2(g)��
��1��д��������Ӧ��ƽ�ⳣ������ʽ_______________��
��2����2L�����ܱ����м�������C��NO������Ӧ����������������ش��������⡣
ʵ���� | �¶�/�� | ��ʼʱNO�����ʵ���/mol | ƽ��ʱN2�����ʵ���/mol |
1 | 700 | 0.40 | 0.09 |
2 | 800 | 0.24 | 0.08 |
����ϱ������ݣ��жϸ÷�Ӧ�ġ�H____0(����������������)��������_________��
���жϸ÷�Ӧ�ﵽƽ���������_______��
A.�����������ܶȺ㶨 B.�����ڸ�����Ũ�Ⱥ㶨
C.������ѹǿ�㶨 D.2v����NO��= v�棨N2��
��3��700��ʱ������2L����㶨���ܱ������г���һ����N2��CO2������Ӧ��N2(g)+CO2(g)![]() C(s)+2NO(g) ������N2��NO���ʵ�����ʱ��仯����������ͼ��ʾ����ش��������⡣
C(s)+2NO(g) ������N2��NO���ʵ�����ʱ��仯����������ͼ��ʾ����ش��������⡣
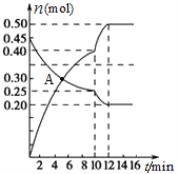
��0��10 min�ڵ�CO2ƽ����Ӧ����v��____________��
��ͼ��A��v(��)___v(��)�����������������������
�۵�10 minʱ�����ı������������_____________��
A���Ӵ��� B������C�����ʵ���
C����СCO2�����ʵ��� D������ E������