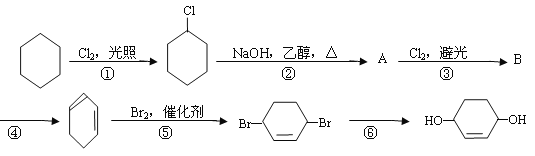
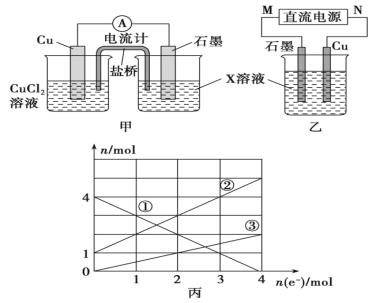
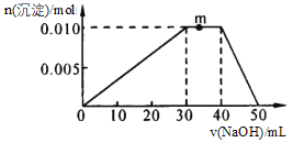
��Ŀ����
����Ŀ����������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
BԪ��ԭ�ӵĺ���p��������s��������1�� |
CԪ��ԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1��738 kJ��mol��1��I2��1 451 kJ��mol��1��I3��7 733 kJ��mol��1��I4��10 540 kJ��mol��1 |
Dԭ�Ӻ�������p���ȫ������� |
EԪ�ص������������������IJ�Ϊ4 |
F��ǰ�������е縺����С��Ԫ�� |
G�����ڱ��ĵ����� |
��1��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���_____������ԭ�ӹ����___��
��2��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�ͼΪ![]()
![]() ��ͬѧ�����ĵ����Ų�ͼΥ����____��
��ͬѧ�����ĵ����Ų�ͼΥ����____��
��3��Gλ��______��______�����۵����Ų�ʽΪ______��
��4������FԪ�ص�ʵ�鷽����_________��
���𰸡�3 ���� ����ԭ�� ��B d 3d54s2 ȡһ�νྻ�IJ�˿������ɫ��������������ɫ��Ȼ��պȡ��Һ��������ɫ���������գ�����ɫ�ܲ����۲��Ƿ�����ɫ
��������
A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ��������������
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�أ���AΪHԪ�أ�
BԪ��ԭ�ӵĺ���p��������s��������1�������Ų�ʽΪ1s22s22p3����BΪNԪ�أ�
Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol��I2=1451kJ/mol��I3=7733kJ/mol��I4=10540kJ/mol����Ԫ�صڶ�������ԶԶС�ڵ��������ܣ���Cλ�ڵ�IIA�壬��ԭ����������B����CΪMgԪ�أ�
Dԭ�Ӻ�������p����ϵ���ȫ������������ԭ����������Mg����DΪPԪ�أ�
EԪ�ص������������������IJ�Ϊ4��EΪ������Ԫ�أ���ԭ����������D����������������VIIA����E��ClԪ�أ�
F��ǰ�������е縺����С��Ԫ�أ����������ǿ��ΪKԪ�أ�
G�����ڱ��ĵ�������λ�ڵ������ڣ���GΪMnԪ�أ�
�ݴ˽��ԭ�ӽṹ�������
��������������AΪHԪ�أ�BΪNԪ�أ�CΪMgԪ�أ�DΪPԪ�أ�ΪClԪ�أ�FΪKԪ�أ�GΪMnԪ�ء�
(1)BΪNԪ�أ�N�Ļ�̬ԭ����������ߵĵ���Ϊ2p���ӣ���������ڿռ���3������ԭ�ӹ���������Σ��ʴ�Ϊ��3�����壻
(2)ijͬѧ����������Ϣ���ƶ�Mg��̬ԭ�ӵĺ�������Ų�Ϊ![]() ����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ�����ʴ�Ϊ������ԭ����
����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ�����ʴ�Ϊ������ԭ����
(3)GΪMnԪ�أ�Mnλ�ڵ�VIIB���������Ϊd���ӣ�Ϊd��Ԫ�أ��۵����Ų�ʽΪ3d54s2���ʴ�Ϊ����B��d��3d54s2��
(4)FΪKԪ�أ�����KԪ�صķ�������ɫ��Ӧ���������Ϊȡһ�νྻ�IJ�˿��ȡһ�νྻ�IJ�˿������ɫ��������������ɫ��Ȼ��պȡ��Һ��������ɫ���������գ�����ɫ�ܲ����۲��Ƿ�����ɫ���ʴ�Ϊ��ȡһ�νྻ�IJ�˿������ɫ��������������ɫ��Ȼ��պȡ��Һ��������ɫ���������գ�����ɫ�ܲ����۲��Ƿ�����ɫ��