��Ŀ����
19����ͼ����ij��þ��2MgO•B2O3•H2O��SiO2������FeS��Ϊԭ�����������Ѿ�����MgSO4������
��֪��Fe3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ����Һ��PH�ֱ�Ϊ3.2��9.7��12.4��
��1�����ڿ���к�CaCO3������ȡ��ʱ���ײ���������ĭʹ���ϴӷ�Ӧ���������ˣ�ʵ�ʲ���������Ӧ��ע����Ƿ�������H2SO4�����������ȡʱ����������M�к���һ���ж������ʣ����Ļ�ѧʽ��H2S����������NaOH��Һ���մ�����
��2��ʵ�����У�������õ��IJ���������©�������������ձ�
��3��������Һ�������ԣ���H3BO3��MgSO4��������Fe3+��Fe2+ �����ʣ������ӡ�ʱ�����Һ���ȼ�������H2O2�ټ���MgO����ȥ��������Fe3+��Fe2+��H2O2��������H2O2+2H++2Fe2+=2Fe3++2H2O�������ӷ���ʽ��ʾ��
��4������ȡ������ٲ��á��ȹ��ˡ���������˵�������¶ȵ����ߣ�H3B03���ܽ�����ߣ���д�����ߡ��������䡱�������͡�����
��5��������2 t ��þ�ࣨ��MgO����������Ϊ25%��������ˮ����þ���������̵IJ���Ϊ 80%��������������ˮ����þ��Ʒ2.46t��
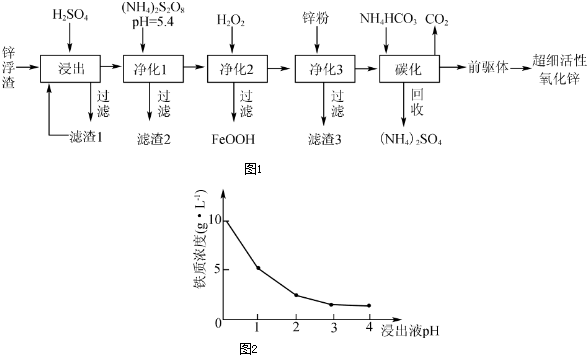
���� ��ij��þ��2MgO•B2O3•H2O��SiO2������FeS��Ϊԭ�����������Լ�����MgSO4�������У���þ��2MgO•B2O3•H2O��SiO2������FeS���м��������ȡ�������ж�����ΪH2S�����ȹ��ˣ��˳�SiO2 ���õ�������Һ�������ԣ���H3BO3��MgSO4��������Fe3+��Fe2+ �����ʣ������ӡ�ʱ�����Һ���ȼ�������H2O2������������Ϊ�����ӣ��ټ���MgO��MgCO3������ҺPH���������ӣ����˽��½ᾧ�õ����ᾧ�壬ĸҺ����ȡ�Ȼ�þ��
��1��CaCO3�����ᷴӦ���ɶ�����̼������ƺ�ˮ������ȡ��ʱ���ײ���������ĭʹ���ϴӷ�Ӧ���������Ҫ�����μ������ᣬ����FeS�����ᷴӦ�����ж�����H2S������ˮ���ᣬ����������������Һ���գ�
��2��������Ƿ�����Һ���壬ʵ������ǹ��ˣ����ݹ���װ�úͲ���ѡ����������
��3��������H2O2��Fe2+����ΪFe3+����MgO����PH��ʹFe3+ת��Ϊ������
��4��H3BO3���ܽ�����¶ȵ����߶�����
��5������þԪ���غ�ͷ�Ӧ�����е���������ʽ���㣬����þ����MgO������������ռ40%�������������MgSO4•7H2O������
��� �⣺��1��CaCO3�����ᷴӦ���ɶ�����̼������ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��CaCO3 ����ĩ��+H2SO4=CaSO4+H2O+CO2��������ȡ��ʱ���ײ���������ĭʹ���ϴӷ�Ӧ�������Ϊ������þ��ң�ʵ�ʲ�����Ӧ��ע����Ƿ����μ���������Һ��������Ӧ���г̶ȣ�����FeS�����ᷴӦ�����ж�����H2S������ˮ���ᣬ����������������Һ���գ�
�ʴ�Ϊ�������������H2S��NaOH��
��2��������Ƿ�����Һ���壬ʵ����������ǹ��ˣ����ݹ���װ�úͲ���ѡ����������Ҫ�У�©�������������ձ����ʴ�Ϊ��©�������������ձ���
��3��������H2O2��Fe2+����ΪFe3+������H2O2�������ǣ�H2O2+2H++2Fe2+=2Fe3++2H2O����MgO����PH��ʹFe3+ת��Ϊ��������ȥ��
�ʴ�Ϊ��MgO��H2O2+2H++2Fe2+=2Fe3++2H2O��
��4������ȡ������ٲ��á��ȹ��ˡ���������˵�������¶ȵ����ߣ�H3B03���ܽ�����¶ȵ����߶���������Ҫ���á��ȹ��ˡ����Է��¶��½�ʱH3BO3����Һ���������ʴ�Ϊ�����ߣ�
��5��������2 t ��þ�ࣨ��MgO����������Ϊ25%��������ˮ����þ���������̵IJ���Ϊ 80%��2t��þ��������þ�����ʵ���Ϊ��$\frac{2t��1{0}^{6}g��25%}{40g/mol}$=1.25��104mol������þԭ���غ㣬����MgSO4•7H2O�����ʵ���Ϊ1.25��104mol������Ϊ246g/mol��1.25��104mol=3.075��106g�����������̵IJ���Ϊ80%������������MgSO4•7H2O������Ϊ��3.075��106g��80%=2.46��106g=2.46t��
�ʴ�Ϊ��2.46t��
���� ���⿼�������ʵķ����ᴿ���漰��ѧ����ʽ����д�����ӷ������Լ���ѡ���Լ�ͼ��ķ����ȣ���Ŀ�Ѷ��еȣ�ע���ͼ��ķ����Ͷ������Ϣ�ķ�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �٢ۢ� | B�� | �ۢڢ� | C�� | �ڢۢ� | D�� | �ڢ٢� |
| ѡ�� | ʵ����� | ���� | ���� |
| A | �ھƾ����ϼ������� | �����ۻ��������� | �۵㣺���������� |
| B | ij���������м���Ba��OH��2��Һ | ������ɫ���� | ����������ֻ��SO42- |
| C | �ò�����պȡŨ����㵽��ɫʯ����ֽ�� | ��ֽ��� | Ũ���������ˮ�� |
| D | ʳ�üӵ��μ���ʳ��KI��Һ���ټ���CCl4������ | �²���Ϻ�ɫ | ��ʳ�üӵ��κ���KIO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 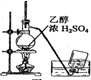 �Ʊ���ϩ | B�� | 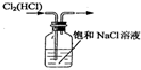 ��ȥCl2�е�HCl | ||
| C�� | 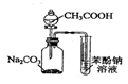 ֤�����ԣ�CH3COOH��H2CO3������ | D�� | 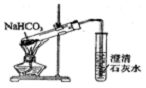 ̽��NaHCO3�����ȶ��� |
| A�� | ˮ��ȴ��0������ʱ��ɱ�������Ϊˮ���Ӵ�Һ�����˹��� | |
| B�� | �����������¶ȱ仯ʱ���ܱ��ֳ���̬�仯 | |
| C�� | �������¶ȣ�ֻ����ѹǿ��Ҳ����ʹ���ʴ���̬��ɹ�̬ | |
| D�� | ������������������Ϊ�������ʵ����ܹ��������� |
I������NO2��һ�ַ��������ü������ԭNO2��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ��mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2
CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H3=-867kJ��mol
���H2=-1160kJ•mol-1
��ʯȼ�ϵ�ȼ�գ����������ʯ��ұ������������������в�����SO2�Ǵ�����SO2����Ҫ��Դ��
��úת��Ϊ˭ÿ���ǽ�úת��Ϊ�ᾧȼ�ϵķ���֮һ����ӦΪ��C��s��+H2O��g��?CO��g��+H2��g��
һ���¶��£���1.0L�ܱ������з���1molC��s����1molH2O��g�����з�Ӧ����Ӧʱ�䣨t����������������ѹǿƽ��p�������ݼ��±�
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.56 | 5.14 | 5.87 | 6.30 | 7.24 | 8.16 | 8.18 | 8.20 | 8.20 |
��1��������Щѡ�����˵���ÿ��淴Ӧ�Ѵ�ƽ��״̬AD
A�����������ܶȲ��ٷ����ı�
B������1molH2O��g����ͬʱ����1molH2
C����H����
D��V����CO��=V����H2��
��2������ѹǿP����ʼѹǿP0��ʾ��Ӧ��ϵ�������ʵ���n�ܣ�n��=$\frac{P}{{P}_{0}}$mol���ɱ������ݼ���ﵽƽ��ʱ����Ӧ��H2O��g����ת���ʦ�=79.82%����ȷ��С�����ڶ�λ��
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ����ֵ�ð����������������£�
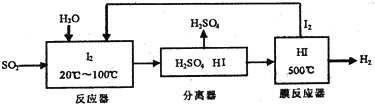
���û�ѧ����ʽ��ʾ�������̷������ܷ�ӦSO2+2H2O=H2SO4+H2
���û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H��Ŀ���ǽ����������Ũ�ȣ�ʹƽ�����������ƶ�
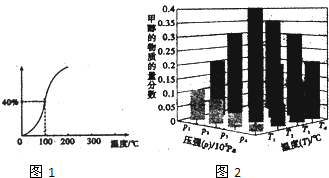
��1����1.0molCH4��2.0molH2O��g��ͨ���ݻ�Ϊ100L�ķ�Ӧ������һ�������·�����Ӧ��
CH4��g��+H2O��g��?CO��g��+3H2��g�������һ����ѹǿ��CH4��ƽ��ת�������¶ȵĹ�ϵ��ͼ1.100��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ7.2��10-5
��2����һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H��O����Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ2��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж�ȷ����CD������ţ�
A��P3��P2��T3��T2 B��P2��P4��T4��T2 C��P1��P3��T1��T3 D��P1��P4��T1��T4
| A�� | �������ķ���ͨʽ��CnH2n-6 ��n��6�� | |
| B�� | ����ͬϵ���Ƿ����н�����һ�������������������� | |
| C�� | ���ͼױ�������ʹKMnO4������Һ��ɫ | |
| D�� | ���ͼױ�������±�ص��ʡ�����ȷ���ȡ����Ӧ |
 ��
��