��Ŀ����
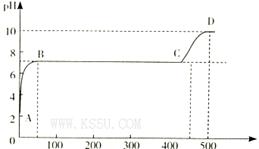
�̲��и�����Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ2Na2O2��2H2O=4NaOH��O2����Ϊ��̽��Na2O2��H2O��Ӧ�Ļ�����ijѧϰ̽��С���ڽ�ʦָ�����������ͼ��ʾװ�ý���ʵ�顣
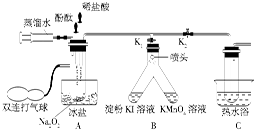
ʵ�鲽�����£�
�ٰ�ͼʾ��װ�����������װ��������Ϊ���ú�װ��ҩƷ��
�ڱ���K1��K2�رգ���ע�����е�����ˮ�����Թ��У���ʱ�Թ��в������������
�ۼ�ѹװ�з�̪�Ľ�ͷ�ιܣ�ʹ��̪�����Թ��У��Թ�����Һ�Ժ�ɫ��
�ܼ�ѹװ��ϡ����Ľ�ͷ�ιܣ�ʹϡ��������Թ��У���ɫ��ʧ���ٵμ�2�Ρ�
����˫����������A���Թ��й�����ʹ�Թ�����Һͨ����ͷ����B��֧���У����ֵ��ۣ�KI��Һ������KMnO4��Һ��ɫ��
��Ѹ�ٴ�K2���ر�K1��������A���Թ��й��������Թ�����Һ����C���Թ���Լ����֮һʱֹͣ������Ȼ������ˮԡ����C���Թ�Ƭ�̣�������ð����������Ϊ������
��ش��������⣺
��1�������ӷ���ʽ��ʾ���ۣ�KI��Һ������ԭ��_____________________________
________________________________________________________________________��
��2�������ӷ���ʽ��ʾKMnO4��Һ��ɫ��ԭ�� ______________________________
________________________________________________________________________��
��3��A���ñ�����ԡ��C������ˮԡ�����÷ֱ���________��______________________��
��4��Na2O2��H2O��Ӧ�Ļ�����____________________���û�ѧ����ʽ��ʾ����
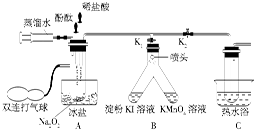
ʵ�鲽�����£�
�ٰ�ͼʾ��װ�����������װ��������Ϊ���ú�װ��ҩƷ��
�ڱ���K1��K2�رգ���ע�����е�����ˮ�����Թ��У���ʱ�Թ��в������������
�ۼ�ѹװ�з�̪�Ľ�ͷ�ιܣ�ʹ��̪�����Թ��У��Թ�����Һ�Ժ�ɫ��
�ܼ�ѹװ��ϡ����Ľ�ͷ�ιܣ�ʹϡ��������Թ��У���ɫ��ʧ���ٵμ�2�Ρ�
����˫����������A���Թ��й�����ʹ�Թ�����Һͨ����ͷ����B��֧���У����ֵ��ۣ�KI��Һ������KMnO4��Һ��ɫ��
��Ѹ�ٴ�K2���ر�K1��������A���Թ��й��������Թ�����Һ����C���Թ���Լ����֮һʱֹͣ������Ȼ������ˮԡ����C���Թ�Ƭ�̣�������ð����������Ϊ������
��ش��������⣺
��1�������ӷ���ʽ��ʾ���ۣ�KI��Һ������ԭ��_____________________________
________________________________________________________________________��
��2�������ӷ���ʽ��ʾKMnO4��Һ��ɫ��ԭ�� ______________________________
________________________________________________________________________��
��3��A���ñ�����ԡ��C������ˮԡ�����÷ֱ���________��______________________��
��4��Na2O2��H2O��Ӧ�Ļ�����____________________���û�ѧ����ʽ��ʾ����
��1��2I����2H����H2O2=I2��2H2O
��2��2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
��3����ֹ���ɵ�H2O2�ֽ⡡ʹH2O2�ֽ�
��4��Na2O2��2H2O=2NaOH��H2O2,2H2O2 2H2O��O2��
2H2O��O2��
��2��2MnO4-��5H2O2��6H��=2Mn2����5O2����8H2O
��3����ֹ���ɵ�H2O2�ֽ⡡ʹH2O2�ֽ�
��4��Na2O2��2H2O=2NaOH��H2O2,2H2O2
 2H2O��O2��
2H2O��O2����ʼ�Թ������������ɣ������̪����Һ���ɫ��˵��Na2O2��H2O��Ӧ�����˼��H2O2��Ȼ������ϡ�����к�NaOH��H2O2��C�зֽ�����O2����1��H2O2��ǿ������������I������2��H2O2ʹKMnO4��Һ��ɫ���������仹ԭ�ԡ���3��A���ñ�����ԡ����ֹ�¶ȹ���H2O2�ֽ⣬C������ˮԡ��ʹH2O2�ֽ⡣��4��Na2O2��H2O��Ӧ����H2O2��H2O2���ȷֽ⡣

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ

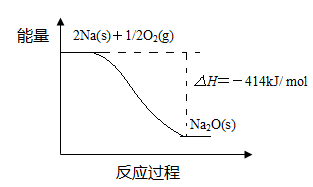
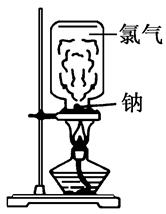
 Ni + 2NaCl����������Ӧʽ��_____��
Ni + 2NaCl����������Ӧʽ��_____��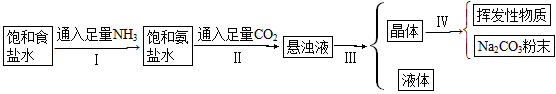

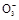
 BaCO3��+H2O)
BaCO3��+H2O)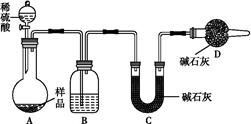
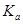 ���±���
���±���