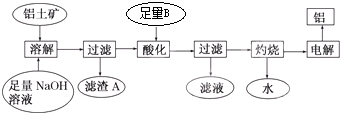
��Ŀ����
10��BaCl2•xH2O����;�㷺�Ļ���������Ʒ���ҹ�Ŀǰ��Ҫ�������������������Mg2+��Fe3+�ȣ���Ӧ����BaCl2•xH2O������������ͼ��ʾ����ش�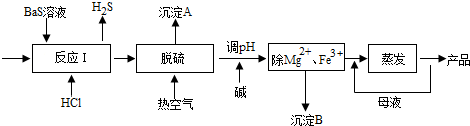
��֪������ʱKsp[Mg��OH��2]=1.8��10-11 Ksp[Fe��OH��3]=4.0��10-38
��1����ӦI�����ɵ�H2S��������ˮ���գ�һ����������������Һ��ͨ�˿������ֿɵõ�������ʹ����Һ������������Ӧ�Ļ�ѧ����ʽΪ2��NH4��2S+O2+2H2O=4NH3•H2O+2S����
��2�������Ȼ�����Һ�к����H2S��HS-�ȣ�Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ���������¶ȣ���С��������Һ�е��ܽ�ȣ����ڴ������⣻����A����Ҫ�ɷ�����
��3���ȿ�������ʱ���в���HS-ת��ΪS2O32-��ʹ��Ʒ�Բ��ܴﵽ����Ҫ�������ữ�����ữ����ʱ�����ӷ���ʽΪS2O32-+2H+=S��+SO2 ��+H2O��
��4������ʱ��ΪʹMg2+��Fe3+��ȫ����������Һ������Ũ��С��1��l05mol•L-1ʱ��Ϊ��������ȫ��������Ӧ����Һ��pH����$-lg\frac{1{0}^{-14}}{\sqrt{1.8��1{0}^{-6}}}$��ֻ����ʽ�����ϣ�
��5��ʵ���Ҳⶨ��Ʒ��x�IJ������£�
��ȷ��ȡ12.23gBaCl2•xH2O��Ʒ������l00mLϡ��������ܽ⣻
�ڱ߽��裬����μ���0��lmol•L-1H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2-3�Σ�
�۽�������ָ�������������Ϊ11.65g��
����BaSO4�����Ƿ�ϴ�Ӹɾ��ķ�����ȡ�����һ��ϴ��Һ�����������ữ����������Һ��������Һ����ϴ�Ӹɾ���������x����ֵΪ2��
���� ��1��H2S��������ˮ���գ���������泥������е������ܽ�������������ʣ��ݴ�д��ѧ����ʽ��
��2��Ԥ�Ⱥ�Ŀ�����ʹ��Һ�¶����ߣ��Ӷ���С��������Һ�е��ܽ�ȣ����ڴ������⣬ͬʱ���������������ܽ������������������ʶ��γɳ�����
��3��S2O32-�����������·�������������ԭ��Ӧ���ɶ�����������ʣ����ݵ���غ��Ԫ���غ��д�����ӷ���ʽ��
��4������Ksp[Mg��OH��2]=1.8��10-11��֪��ҪʹMg 2+������ȫ����Һ��c��OH-��=$\sqrt{\frac{1.8��1{0}^{-11}}{1��1{0}^{-5}}}$mol/L=$\sqrt{1.8��1{0}^{-6}}$mol/L����PHֵΪ$-lg\frac{1{0}^{-14}}{\sqrt{1.8��1{0}^{-6}}}$������Ksp[Fe��OH��3]=4.0��10-38ҪʹFe 3+������ȫ����Һ��c��OH-��=$\root{3}{\frac{4.0��1{0}^{-38}}{1.0��1{0}^{-5}}}$mol/L=$\root{3}{4.0��1{0}^{-33}}$mol/L�����Ե�Mg 2+������ȫʱFe 3+���ѳ�����ȫ���ݴ��жϣ�
��5��BaSO4�����ǴӺ��������ӵ���Һ�������ģ����Կ���ͨ��������������Ƿ����������жϳ���ϴ���Ƿ�ɾ����������е�������ݿ�֪��12.23gBaCl2•xH2O��Ʒ������BaSO4��������Ϊ11.65g�����ݹ�ϵʽBaCl2•xH2O��BaSO4�б���ʽ�ɼ����x��ֵ��
��� �⣺��1��H2S��������ˮ���գ���������泥������е������ܽ�������������ʣ���Ӧ�Ļ�ѧ����ʽΪ2��NH4��2S+O2+2H2O=4NH3•H2O+2S����
�ʴ�Ϊ��2��NH4��2S+O2+2H2O=4NH3•H2O+2S����
��2��Ԥ�Ⱥ�Ŀ�����ʹ��Һ�¶����ߣ��Ӷ���С��������Һ�е��ܽ�ȣ����ڴ������⣬ͬʱ���������������ܽ������������������ʶ��γɳ�����
�ʴ�Ϊ�������¶ȣ���С��������Һ�е��ܽ�ȣ����ڴ������⣻��
��3��S2O32-�����������·�������������ԭ��Ӧ���ɶ�����������ʣ���Ӧ�����ӷ���ʽΪS2O32-+2H+=S��+SO2 ��+H2O��
�ʴ�Ϊ��S2O32-+2H+=S��+SO2 ��+H2O��
��4������Ksp[Mg��OH��2]=1.8��10-11��֪��ҪʹMg 2+������ȫ����Һ��c��OH-��=$\sqrt{\frac{1.8��1{0}^{-11}}{1��1{0}^{-5}}}$mol/L=$\sqrt{1.8��1{0}^{-6}}$mol/L����PHֵΪ$-lg\frac{1{0}^{-14}}{\sqrt{1.8��1{0}^{-6}}}$������Ksp[Fe��OH��3]=4.0��10-38ҪʹFe 3+������ȫ����Һ��c��OH-��=$\root{3}{\frac{4.0��1{0}^{-38}}{1.0��1{0}^{-5}}}$mol/L=$\root{3}{4.0��1{0}^{-33}}$mol/L�����Ե�Mg 2+������ȫʱFe 3+���ѳ�����ȫ������Ӧ����Һ��pH����$-lg\frac{1{0}^{-14}}{\sqrt{1.8��1{0}^{-6}}}$���ϣ�
�ʴ�Ϊ��$-lg\frac{1{0}^{-14}}{\sqrt{1.8��1{0}^{-6}}}$��
��5��BaSO4�����ǴӺ��������ӵ���Һ�������ģ����Կ���ͨ��������������Ƿ����������жϳ���ϴ���Ƿ�ɾ�������Ϊȡ�����һ��ϴ��Һ�����������ữ����������Һ��������Һ����ϴ�Ӹɾ����������е�������ݿ�֪��12.23gBaCl2•xH2O��Ʒ������BaSO4��������Ϊ11.65g��
���ݹ�ϵʽBaCl2•xH2O��BaSO4
208+18x 233
12.23 11.65
$\frac{208+18x}{12.23}=\frac{233}{11.65}$������x=2
�ʴ�Ϊ��ȡ�����һ��ϴ��Һ�����������ữ����������Һ��������Һ����ϴ�Ӹɾ���2��
���� ���⿼����BaCl2•2H2O�Ʊ�ʵ�鷽������Ʒ�������Ŀ�Ѷ��еȣ�Ϊ�߿����ȵ㣬��ȷ�����Ʊ����̡���Ӧԭ��Ϊ���ؼ����漰֪ʶ��϶࣬��һ�����ۺ��ԣ������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

| A�� | NO��O2��Ӧ����NO2 | B�� | NH3������������NO | ||
| C�� | ��NH3��̼狀���� | D�� | N2��H2��һ�������ºϳɰ� |
| A�� | ��ǿ�ȡ������� | B�� | ���ȡ������� | C�� | ��ʴ���ȶ��Ժ� | D�� | ���硢�����Ժ� |
| A�� | H2O | B�� | SO32- | C�� | NH3 | D�� | CCl4 |
| A�� | MgCl2 | B�� | Na2O2 | C�� | KHSO4 | D�� | NH4Cl |
| A�� | 1 �� | B�� | 2 �� | C�� | 3 �� | D�� | 4 �� |