��Ŀ����
��֪H2(g)+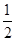 O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������
O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������
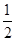 O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������
O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������| A��H2��ȼ����Ϊ241.8kJ/mol |
| B��2H2(g)+O2(g)=2H2O(g)����H=-483.6kJ/mol |
| C��1mol H2��ȫȼ������Һ̬ˮ�ų�����������24l.8kJ |
| D���Ͽ�1molH2O�Ļ�ѧ�����յ����������ڶ���lmolH2��0.5molO2�Ļ�ѧ�������յ������� |
A
��25���϶�,101 kPaʱ,1 mol��ȼ����ȫȼ�������ȶ��Ļ�����ʱ���ų�������,���������ʵ�ȼ����. A��ͨ����ɿ�֪���ɵ�H2O(g)�����ߣ����ȶ����ɽ�һ����������Һ̬ˮ�����H2��ȼ����Ӧ����241.8kJ/mol��A�����

��ϰ��ϵ�д�
�����Ŀ
 ��
��
 OH��)Ϊ
OH��)Ϊ