��Ŀ����
����Ŀ������������(H2N2O2)��һ�ֶ�Ԫ�ᣬ��������N2O���塣
(1)�����������е�Ԫ�صĻ��ϼ�Ϊ________��
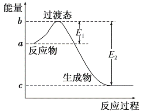
(2)�����£���0.01 mol��L��1��NaOH��Һ�ζ�10 mL 0.01 mol��L��1H2N2O2��Һ�������ҺpH��NaOH��Һ����Ĺ�ϵ��ͼ��ʾ��
��д��H2N2O2��ˮ��Һ�еĵ��뷽��ʽ��____________________________��
��c��ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ_______________________________��
��b��ʱ��Һ��c(H2N2O2)________(�������������������ͬ)c(N2O![]() )��
)��
��a��ʱ��Һ��c(Na��)________c(HN2O)��c(N2O![]() )��
)��
(3)��������Һ����������������Һ��ϣ����Եõ���ɫ����������������������÷�ɢϵ�еμ���������Һ������ɫ�����ͻ�ɫ��������ʱ����ɢϵ��![]() ��________��[��֪Ksp(Ag2N2O2)��4.2��10��9��Ksp(Ag2SO4)��1.4��10��5]
��________��[��֪Ksp(Ag2N2O2)��4.2��10��9��Ksp(Ag2SO4)��1.4��10��5]
���𰸡���1H2N2O2![]() HN2O
HN2O![]() ��H��c(Na��)��c(N2O
��H��c(Na��)��c(N2O![]() )��c(OH��)��c(HN2O
)��c(OH��)��c(HN2O![]() )��c(H��)>>3.0��10��4
)��c(H��)>>3.0��10��4
��������
��1������H2N2O2��H��OԪ�صĻ��ϼ۷ֱ�Ϊ+1��-2���ܻ��ϼ�֮��Ϊ0���м���NԪ�صĻ��ϼۣ�
��2���ٸ���ͼ�����֪����0.01 mol��L��1H2N2O2��Һ��pH=4.3��˵��0.01 mol��L��1H2N2O2��Һ��Ϊ��Ԫ���ᣬ��Ԫ����ֻҪ�Ե�һ������Ϊ�����ݴ�д������뷽��ʽ��
��c�����20mL��ͬŨ�ȵ�NaOH��Һ����Ӧ������ΪNa2N2O2��HN2O2-����ˮ�⣬��Һ�ʼ��ԣ���ϵ���غ��жϸ�����Ũ�ȴ�С��
��c������ΪNaHN2O2����Һ�ʼ��ԣ���HN2O2-��ˮ��̶ȴ��������̶ȣ�
��a��ʱ��Һ��pH=7����c(OH-)=c(H+)�����ݵ���غ����֪��c(Na��)=c(HN2O)��2c(N2O![]() )��
)��
��3������![]() =
=![]() �����ߵ��ܶȻ����м�����
�����ߵ��ܶȻ����м�����
��1��H2N2O2������H�Ļ��ϼ�Ϊ+1��OԪ�صĻ��ϼ�Ϊ-2����NԪ�صĻ��ϼ�Ϊx�������ܻ��ϼ�֮��Ϊ0����֪����2x+(+1)![]() ������ó���x=1����NԪ�صĻ��ϼ�Ϊ+1��
������ó���x=1����NԪ�صĻ��ϼ�Ϊ+1��
��ˣ�������ȷ���ǣ�+1��
��2���ٸ���ͼ�����֪��������������Һ���Ϊ0ʱ��0.01 mol��L��1H2N2O2��Һ��pH=4.3��˵��H2N2O2Ϊ��Ԫ���ᣬ��Ԫ����ֻҪ�Ե�һ������Ϊ����������뷽��ʽΪ��H2N2O2![]() HN2O
HN2O![]() ��H����
��H����
��ˣ�������ȷ���ǣ�H2N2O2![]() HN2O
HN2O![]() ��H����
��H����
��c�����20mL��ͬŨ�ȵ�NaOH��Һ����Ӧ������ΪNa2N2O2��HN2O2-����ˮ�⣬��Һ�ʼ��ԣ���c(OH-)>c(H+)����Ϊ��Һ�����������ӻ�����ˮ�ĵ��뼰HN2O![]() ��ˮ�⣬��c(OH��)��c(HN2O
��ˮ�⣬��c(OH��)��c(HN2O![]() )����Һ������Ũ�ȴ�СΪ��c(Na��)��c(N2O
)����Һ������Ũ�ȴ�СΪ��c(Na��)��c(N2O![]() )��c(OH��)��c(HN2O
)��c(OH��)��c(HN2O![]() )��c(H��)��
)��c(H��)��
��ˣ�������ȷ���ǣ�c(Na��)��c(N2O![]() )��c(OH��)��c(HN2O
)��c(OH��)��c(HN2O![]() )��c(H��)��
)��c(H��)��
��c������ΪNaHN2O2����Һ��pH>7��˵����Һ��ʾ���ԣ���HN2O![]() ��ˮ��̶ȴ��������̶ȣ�����c(H2N2O2)>c(N2O
��ˮ��̶ȴ��������̶ȣ�����c(H2N2O2)>c(N2O![]() )��
)��
��ˣ�������ȷ���ǣ�����
�ܸ���ͼ�����֪����a��ʱ��Һ��pH=7����Һ�����ԣ���c(OH-)=c(H+)�����ݵ���غ����֪��c(Na��)=c(HN2O)��2c(N2O![]() )������c(Na��)>c(HN2O)��c(N2O
)������c(Na��)>c(HN2O)��c(N2O![]() )��
)��
��ˣ�������ȷ���ǣ�����
��3�������ֳ�������ʱ��![]() =
=![]() =
=![]() =3.0��10��4��
=3.0��10��4��
��ˣ�������ȷ���ǣ�3.0��10��4��

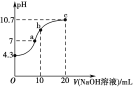
����Ŀ����һ���¶��£�������X������Y��0.16 mol����10 L�����ܱ������У�������ӦX(g)+Y(g)![]() 2Z(g)����H<0��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
2Z(g)����H<0��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ���������±���
t/min | 2 | 4 | 7 | 9 |
n(Y)/mol | 0.12 | 0.11 | 0.10 | 0.10 |
����˵����ȷ����
A. ��Ӧǰ2 min��ƽ������v(Z)=2.0��10-3 mol��(L��min)-1
B. �����������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰv(��)>v(��)
C. ���¶��´˷�Ӧ��ƽ�ⳣ��K=1.44
D. �����������䣬�ٳ���0.2 mol Z��ƽ��ʱX�������������
����Ŀ�������Դ��ȱ���⣬��ҵ������Ӧ�������û�ѧ�ܡ�
(1)25 �桢1.01��105 Paʱ��ʵ���ã�4 g������O2����ȫȼ������Һ̬ˮ���ų�572 kJ�����������ʾH2��ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________��
(2)�ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡�
��ѧ�� | H��H | N��H | N��N |
����/kJ��mol��1 | 436 | a | 945 |
��֪��N2(g)��3H2(g)===2NH3(g)����H����93 kJ��mol��1���Ը��ݱ������м������ݼ���a����ֵ____________��
(3)��֪��C(s��ʯī)��O2(g)===CO2(g)��H1����393.5 kJ��mol��1����
2H2(g)��O2(g)===2H2O(l)��H2����571.6 kJ��mol��1����
2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)��H3����2599 kJ��mol��1����
���ݸ�˹���ɣ����㷴Ӧ2C(s��ʯī)��H2(g)===C2H2(g)����H��________��
����Ŀ���״�����Ҫ�Ĺ�ҵԭ�ϡ�ú������������ú̿��ȡˮú���Ӷ��ϳɼ״���CO(g)��2H2(g)![]() CH3OH(g)��
CH3OH(g)��
��֪�ٳ�ѹ�·�Ӧ�������仯��ͼ��ʾ��
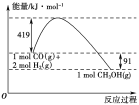
��
��ѧ�� | H��H | H��O | O===O |
����kJ/mol | 436 | x | 496 |
��CO(g)��1/2O2(g)===CO2(g)��H����280 kJ/mol��
H2(g)��1/2O2(g)===H2O(l)����H����284 kJ/mol
H2O(l)===H2O(g)����H����44 kJ/mol
��ش��������⣺
(1)��д����ʾ��̬�״�ȼ���ȵ��Ȼ�ѧ����ʽ_______________________��
(2)H��O���ļ���xΪ________ kJ/mol��
(3)�״�����ֽ�ΪCO��H2��������ķ�Ӧ�Ļ��Ϊ________ kJ/mol��
(4)��____(����¡����¡�)�����������CO��H2�Ʊ��״��ķ�Ӧ�Է����С�
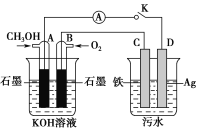
(5)ij��ȤС��ͬѧ���ü״�ȼ�ϵ��̽���縡ѡ��������ˮ��һ�ַ�ʽ��������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe(OH)3������Fe(OH)3���������ԣ�������������������������о���ˮ�����á�װ����ͼ��ʾ��
�ס�������������
��д���׳ص�A���缫��Ӧʽ��__________________________��
�����ҳ�ʵ��ʱ��ˮ������Ũ�Ƚ�С�����������ϲ��ˮЧ�����ã���ʱӦ����ˮ�м���������________��
A��H2SO4 B��BaSO4 C��Na2SO4 D��NaOH E��CH3CH2OH