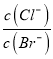��Ŀ����
����Ŀ�������Ҫ����գ�
��1��ˮ�ĵ�������Һ�������������ء�
�ٳ����£�ij����ˮ��Һ��pH=3�����е�c(OH-)=__________��
�ڳ�����,Ũ�Ⱦ�Ϊ0.1 molL-1��CH3COOH��Һ��CH3COONa��Һ��,ˮ�ĵ���̶ȴ�С��ϵ�ǣ�ǰ��_____________���ߣ�����>����<������=������
�۳����£�Ũ�Ⱦ�Ϊ0.1 molL-1��NaX��NaY��������Һ����pH�ֱ�Ϊ8 ��10����HX ��HY������ǿ����ϵ�ǣ�HX_________HY(����>������<��)��
��2���о���ѧ��������ת����װ�á����̺�Ч�ʵĿ�ѧ�������绯ѧ��
��ijԭ���װ������ͼ����

��ԭ��صĸ�����__________������Zn������Cu�����������ĵ缫��ӦʽΪ_______________________��
���ò��缫���ij�����Ȼ���(XCl2)����Һ�����ռ���2.24 LCl2����״����ʱ����������6.4 g����ý��������ԭ������Ϊ_______________________________��
���𰸡� 1.0��10-11 < > Zn Cu2++2e-==Cu 64
����������1���ٳ����£�ij����ˮ��Һ��pH=3,�����е�c(H +)=1.0��10-3mol/L����ˮ�����ӻ������������c(OH -)=1.0��10-11mol/L;
��CH3COOH����������ӿ�������ˮ�ĵ��룬CH3COONaˮ����Դٽ�ˮ�ĵ��롣�����£�Ũ�Ⱦ�Ϊ0.1mol/L��CH3COOH��Һ��CH3COONa��Һ�У�ˮ�ĵ���̶ȴ�С��ϵ����ǰ��<���ߣ�
�۳����£�Ũ�Ⱦ�Ϊ0.1mol/L��NaX��NaY ��������Һ����pH �ֱ�Ϊ8��10��˵��Y-ˮ��̶Ƚϴ���HX��HY������ǿ����ϵ��HX>HY��
��2������ͼ��֪����ԭ��صĸ�����Zn�������ĵ缫��ӦʽΪCu2++2e- =Cu.
���ò��缫���ij�����Ȼ���(XCl2)����Һ�����ռ���2.21LCl2(��״��)ʱ����·��ת�Ƶ��ӵ����ʵ���Ϊ0.2mol����������6.4g���������ĵ缫��ӦX2++2e- =X��֪����������0.1mol X ���ý��������ԭ������Ϊ![]() 64��
64��

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�