��Ŀ����
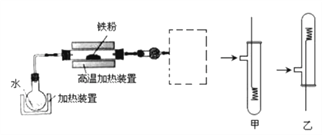
����Ŀ��ij������Ա��Ƴ�������������Ҫ�ɷ�Fe2O3������������SiO2�����ʣ������õ����̣�����ͼ���������е���Һ�������ѭ�������������Ʊ���������ĩ��

��1��Ϊ�˼ӿ췴Ӧ�ٵķ�Ӧ���ʣ����˽��衢�ʵ������¶ȡ���������������ɲ��õĴ�ʩ��____________________________��д��һ�㼴�ɣ���
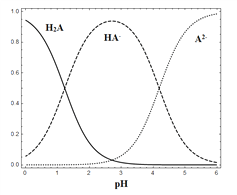
��2����Ӧ����Ҫ����������ԭ��������Fe3+ת��ΪFe2+�����¶�T1��T2��T1��T2���½��и÷�Ӧ��ͨ�������ͬʱ������Һ��pH������pH��ʱ��ı仯��������ͼ��ʾ���ó����ۣ��÷�Ӧ���¶Ȳ��˹��ߡ�

����֪��Ϣ��pH= -lgc(H+)����0.1mol/L�������pH= -lg0.1=1��������������ͨ��SO2��������ԭ��ʱ���������ӷ���ʽ���ʵ�����������pH�½���ԭ����__________________________��
����ͬʱ���ڣ�T1�¶��£���Һ��pH���ߵ�ԭ����____________________________________��
��3����������ѭ��ʹ�õ������� _____________��
��4������һ������FeO��Fe2O3����Ҫ��ѧ����ȷ�����к�������������Ӧѡ����Լ���________������ѡ����ĸ��
A. ���ᡢ���軯����Һ B. ϡ���ᡢ���Ը��������Һ
C. ϡ���ᡢ���軯����Һ D. ϡ���ᡢ���軯����Һ
��5��Ϊ�ⶨ��Ӧ�ٺ���Һ��Fe3����Ũ���Կ��Ƽ���SO2������ʵ�鲽��Ϊ��ȷ��ȡ20.00ml�ķ�Ӧ����Һ��ϡ�ͳ�100mL��Һ��ȡ10.00 mL��Һ������������KI�����2��3�ε�����Һ����0.50mol/L��Na2S2O3��Һ��ⷴӦ������Ӧǡ����ȫ����ʱ��������Na2S2O3��Һ20.00 mL����֪��2Na2S2O3 + I2 �� Na2S4O6 + 2NaI���Լ���ԭ��Һ��Fe3�������ʵ���Ũ��(д���������)��______________
���𰸡� �ʵ���������Ũ�� SO2+2Fe3++2H2O=SO42- +2Fe2++4H+ ��������H+��H+Ũ�ȴ�������ǿ��pH�½� ����֪֪��T1��T2���¶ȸߣ������ܽ���½������뷴Ӧ��SO2�٣���Һ�е�H+Ũ��С��pH�� H2SO4 B 5.00mol/L
����������1��Ϊ�˼ӿ췴Ӧ�ٵķ�Ӧ���ʣ����˽��衢�ʵ������¶ȡ���������������ɲ��õĴ�ʩ���ʵ���������Ũ�ȣ���ȷ�𰸣��ʵ���������Ũ�ȡ�
��2���������������ᷴӦ������������Fe3+���������ԣ��Ѷ�������������������ӣ������������ᣬ������ǿ��pH�½�����ȷ�𰸣�SO2+2Fe3++2H2O=SO42- +2Fe2++4H+ ��������H+��H+Ũ�ȴ�����ǿ��pH�½���
������֪��֪��T1��T2���¶ȸߣ������ܽ���½������뷴Ӧ��SO2�٣���Һ�е�H+Ũ��С��pH�ߣ���ȷ�𰸣� ����֪��֪��T1��T2���¶ȸߣ������ܽ���½������뷴Ӧ��SO2�٣���Һ�е�H+Ũ��С��pH�ߡ�
��3���ܽ�������ʱ�����������Ӧ���ɵ������ӰѶ�������������Ϊ���ᣬ���Ը�������ѭ��ʹ�õ�������H2SO4 ����ȷ���� H2SO4��
��4������FeO��Fe2O3�Ļ�������ȼ���ϡ������Һ����Ӧ�����������������������ټ������Ը��������Һ����Һ��ɫ��֤����Һ��������������ԭ�����к���FeO���������������ܽ��������ΪFeO�ܹ�����������Ϊ�����ӣ�Ӱ���������ӵļ�������ȷѡ��B��
��5�����ݷ�Ӧ2Na2S2O3 + I2 �� Na2S4O6 + 2NaI���Լ�����������2Na2S2O3 --I2���ⵥ�ʵ���=0.5��20��10-3/2=5��10-3 mol���и��ݷ�Ӧ��2Fe3++2I-= I2+ 2Fe2+��֪��2Fe3+--I2��Fe3+����Ϊ10-2mol����֪10.00 mL��Һ��Fe3+����Ϊ10-2mol����100mL��Һ�к���Fe3+����Ϊ10-1mol����20.00ml��Һ��Fe3�������ʵ���Ũ��Ϊ10-1/0.02=5mol/L����ȷ����5.00mol/L��