��Ŀ����
�ҹ�����ר�Һ�°����ĺ����Ƽ�Ļ�ѧԭ���ǽ�������̼ͨ�백ˮ���Ȼ��Ʊ�����Һ�У��仯ѧ��Ӧ����ʽΪ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
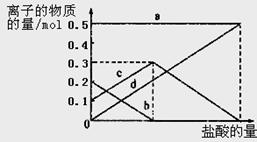
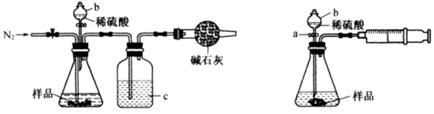
��1����ʵ��������������ԭ���ӷ�Ӧ������Һ�з����̼�����ƾ��壬Ӧѡ������װ���е� ��
��2��ʵ������̼�����ƾ����У����ܺ��е�����������Cl����NH4+��ʵ���Ҽ���Cl����ѡ�õ��Լ��ǡ���������һ���������ӵķ����� ������ţ���
��3��̼�����ƾ������ȷֽ�ɵõ�����仯ѧ��Ӧ����ʽΪ
��1����ʵ��������������ԭ���ӷ�Ӧ������Һ�з����̼�����ƾ��壬Ӧѡ������װ���е� ��

��2��ʵ������̼�����ƾ����У����ܺ��е�����������Cl����NH4+��ʵ���Ҽ���Cl����ѡ�õ��Լ��ǡ���������һ���������ӵķ����� ������ţ���
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B��������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| C��������������Һ�����ȣ������̪�Լ� |
| D��������������Һ�����ȣ�������ɫʯ���Լ� |
��1��B ��2��AgNO3 HNO3 B ��3��2NaHCO3 Na2CO3��CO2����H2O
Na2CO3��CO2����H2O
 Na2CO3��CO2����H2O
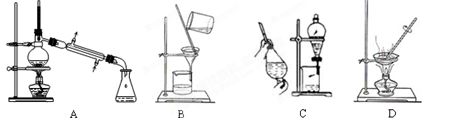
Na2CO3��CO2����H2O�����������1��Aװ�������ڷ��뻥�ܵķе㲻ͬ��Һ������װ�ã�Bװ�������ڷ��������ԵĹ���������Ե�Һ�������װ�ã�Cװ��Ϊ��ȡ��Һװ�ã�Dװ��Ϊ�����ᾧװ�á��ʸ��ݺ����Ƽ��ȡ�����ʵ��ص�Ӧ��ѡ�ù���װ�á���ΪBװ�á���2��ʵ�������������������������Ag+��Cl-��Ӧ�����Ȼ�������������Cl-��Ϊ���ų��������ӵĸ���Ҫ��ϡ���ᡣ����ѡ���Լ�ΪAgNO3��HNO3����NH3��ʵ����������Ψһ��ʹʪ��ĺ�ɫʯ����ֽ���������壬�ʼ���NH4+ʱ�ɽ�NH4+ת��ΪNH3��������Һ�м�������������Һ�����ȣ������Թܿ���ʪ��ĺ�ɫʯ����ֽ���顣����ֽ��Ϊ��ɫ����֤�������������к���NH3��ԭ��Һ�к���NH4+����ѡB����3��̼�����Ʋ��ȶ����ȷֽ⡣�ֽ�Ļ�ѧ����ʽΪ��2NaHCO3
 Na2CO3��CO2����H2O������NH4+�ļ����Լ�̼�����ƺ�̼����֮���ת����֪ʶ��
Na2CO3��CO2����H2O������NH4+�ļ����Լ�̼�����ƺ�̼����֮���ת����֪ʶ��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
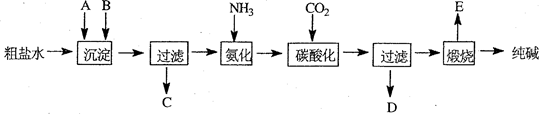
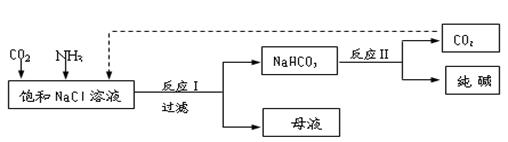
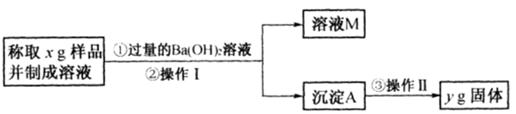
 NaHCO3��+NH4Cl������ĸҺ�����ַ�����
NaHCO3��+NH4Cl������ĸҺ�����ַ�����