��Ŀ����
3��Ǧ���仯������������ء������豸��X���߷������ϵȣ��ش��������⣺��1��Ǧ��̼��ͬ��Ԫ�أ���̼��4�����Ӳ㣬�� PbO2�����Ա�CO2�����������ǿ������������
��2��PbO2��Ũ���Ṳ�����ɻ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪPbO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$PbCl2+Cl2��+2H2O��
��3����Ŧ�Ʒ�����Ǧ������ط�Ӧ���Ȼ�ѧ����ʽ���£�
2PbS��s��+3O2��g��=2PbO��s��+2SO2��g����H=a kJ•mol-1
PbS��s��+2PbO��s��=3Pb��s��+SO2��g����H=b kJ•mol-1
PbS��s��+PbSO4��s��=2Pb��s��+2SO2��g����H=c kJ•mol-1
��Ӧ3PbS��s��+6O2��g��=3PbSO4��s����H=2a+2b-3ckJ•mol-1���ú�a��b��c�Ĵ���ʽ��ʾ����
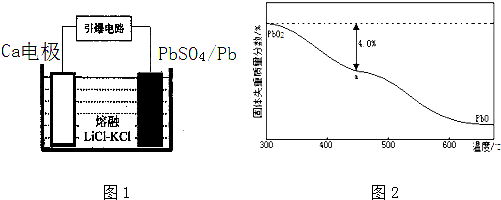
��4�������������������Ĺ�����Դͨ��Ca/PbSO4�ȵ�أ���װ����ͼ1��ʾ���õ�������ĵ缫��ӦʽΪPbSO4+2e-=SO42-+Pb��
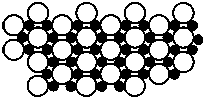
��5��PbO2�ڼ��ȹ��̷����ֽ��ʧ��������ͼ2��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%����$\frac{��Ʒ��ʼ����-a���������}{��Ʒ��ʼ����}$��100%���IJ������壬��a�������ɱ�ʾΪPbOx��mPbO2•nPbO�������x=1.4��m��n=2��3��
���� ��1��Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ��
��2��PbO2��Ũ���Ṳ�����ɻ���ɫ���壬˵�����߷�Ӧ����������ͬʱ�����Ȼ�Ǧ��ˮ��
��3�����ݸ�˹������дĿ���Ȼ�ѧ����ʽ��
��4��ԭ��ص缫��Ӧ����ʽ����д�����ȸ���װ��ͼ���ж���������Ȼ����д�����缫��Ӧ����ʽ��
��5����Ʒʧ��ʧȥ��Ϊ��Ԫ�أ�����ԭ���غ���Oԭ�Ӻ�Pbԭ�ӵı�ֵ��
��� �⣺��1��C��Pb����ͬһ����Ԫ�أ�Ԫ�طǽ�����Խǿ��������������ˮ��������Խǿ���ǽ�����C��Pb������Ԫ������������ˮ��������Pb�ı�C�����������ʴ�Ϊ������
��2��PbO2��Ũ���Ṳ�����ɻ���ɫ���壬˵�����߷�Ӧ����������ͬʱ�����Ȼ�Ǧ��ˮ����Ӧ����ʽΪPbO2+4HCl��Ũ��=PbCl2+Cl2��+2H2O��
�ʴ�Ϊ��PbO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$PbCl2+Cl2��+2H2O��
��3����֪����2PbS��s��+3O2��g��=2PbO��s��+2SO2��g����H=a kJ•mol-1
��PbS��s��+2PbO��s��=3Pb��s��+SO2��g����H=b kJ•mol-1
��PbS��s��+PbSO4��s��=2Pb��s��+2SO2��g����H=c kJ•mol-1
���١�2+�ڡ�2-�ۡ�3�ɵ÷�Ӧ3PbS��s��+6O2��g��=3PbSO4��s��
���ݸ�˹���ɡ�H=2��H1+2��H2-3��H3=��2a+2b-3c��kJ•mol-1
�ʴ�Ϊ��2a+2b-3c��
��4������װ��ͼ���ж�����ΪPbSO4������ԭ��Ӧ���ʴ�ΪPbSO4+2e-=SO42-+Pb���ʴ�Ϊ��PbSO4+2e-=SO42-+Pb��
��5����a�������ɱ�ʾΪPbOx������PbO2$\frac{\underline{\;\;��\;\;}}{\;}$PbOx+$\frac{2-x}{2}$O2����
$\frac{2-x}{2}$��32=239��4.0%��x=1.4��
�����ΪmPbO2•nPbO������ԭ���غ�ã�Oԭ�Ӻ�Pbԭ�ӵı�ֵ=$\frac{2m+n}{m+n}$=1.4����m��n=2��3��
�ʴ�Ϊ��1.4��2��3��
���� ���⿼��̼��Ԫ�����ʣ���Ŀ�Ѷ��еȣ��漰��˹���ɵ�Ӧ�á����ӷ�Ӧ�����ԭ����֪ʶ�㣬ע�⣨3���������������缫��Ӧʽ����д��ע�⣨4���еļ��㣬Ϊ�״��㣮

| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ����������ͨ��������� | B�� | ����ѹǿ | ||
| C�� | ���� | D�� | ������� |
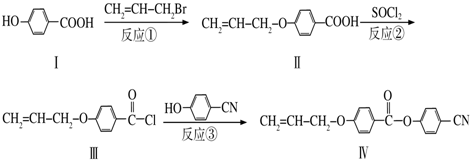
 2001��3�·�����þ��39Kʱ�ʳ����ԣ��� ��������Գ�����ʶ������̱�������þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ�����������У���ͼ�Ǹþ����ۿռ���ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϣ�������ͼȷ����þ�Ļ�ѧʽΪ��������
2001��3�·�����þ��39Kʱ�ʳ����ԣ��� ��������Գ�����ʶ������̱�������þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ�����������У���ͼ�Ǹþ����ۿռ���ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϣ�������ͼȷ����þ�Ļ�ѧʽΪ��������| A�� | MgB | B�� | MgB2 | C�� | Mg2B | D�� | MgB6 |

| A�� | ԭ�Ӱ뾶��Y��Z��X | |
| B�� | ��Y��������Ϊ�����������Z��һ�ֵ����ڿ���������ȼ | |
| C�� | ��X����̬�⻯����Z������������ˮ�����ܷ�Ӧ�������ɵ��ν���һ�� | |
| D�� | ��Y��Z�ĺ˵����֮��ΪX��4������X��Y��Z���⻯����ӵ����幹�ͷֱ�Ϊ��V�Σ������Σ�ֱ���� |
 ��һ�֣�
��һ�֣� Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��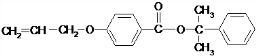 ��
��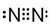 ����2��H2O2��
����2��H2O2�� ��
�� ����4��CO2��
����4��CO2�� ��
��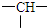 �������ܵĽṹ��Ŀ�ǣ�������
�������ܵĽṹ��Ŀ�ǣ�������