��Ŀ����
15��ͼ1Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ��û�ѧ����ش��������⣺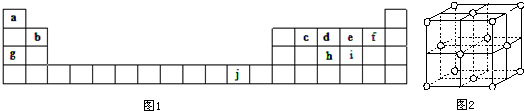
��1��д��Ԫ��j�Ļ�̬ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d104s1����ca4�����У�Ԫ��c���ӻ�Ϊsp3�����ӵĿռ乹��Ϊ�������壮
��2��ci2���ӵĵ���ʽΪ
 ��ci2��ce2�Ƚϣ��е�ϸߵ���CS2��д����ʽ����
��ci2��ce2�Ƚϣ��е�ϸߵ���CS2��д����ʽ������3��da3��ha3��ȣ�da3�ķе�Ҫ�ߵö࣬��ԭ����NH3���Ӽ��γ����
��4����һ�����ܣ�h��i���縺�ԣ�g��b�����������������=������
��5��д��ce2���ӵ�һ�ֵȵ�����Ļ�ѧʽCS2
��6������ˮ���뵽j����������Һ�У��Ȳ�����ɫ������Ȼ��������ܽⲢ�õ�����ɫ��Һ��������ɫ��������[Cu��NH3��4]2+��д�����ӷ��ŵĻ�ѧʽ����
��7��j�Ľ�������ľ�����ͼ2��ʾ����һ��������jԭ�ӵĸ�����4����
���� ����Ԫ�������ڱ��е�λ�ÿ�֪��aΪHԪ�ء�bΪBeԪ�ء�cΪCԪ�ء�dΪNԪ�ء�eΪOԪ�ء�fΪFԪ�ء�gΪNaԪ�ء�hΪPԪ�ء�iΪSԪ�ء�jΪCuԪ�أ�
��1��jΪCuԪ�أ�ԭ�Ӻ��������ĿΪ29�����ݺ�������Ų�������д��aΪHԪ�ء�cΪCԪ�أ���ca4����ΪCH4��Ԫ��CΪsp3�ӻ���
��2��CO2��CS2�Ƚϣ����ӽṹ���ƣ�Ϊ���Ӿ��壬���Ӿ�����۷е�ߵ�ȡ������Է��������Ĵ�С��
��3��da3��ha3��ȣ�NH3�ķе�Ҫ�ߵö�ԭ��ΪNH3���Ӽ��γ������
��4��ͬ����������ҵ�һ�����ܳ��������ƣ���Ԫ��ԭ�Ӹ�������ڰ�����ȫ����ȫ��ʱ�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�
������Խ�����縺��Խ��
��5��ԭ��������ȣ��۵���������ȵ�����Ϊ�ȵ����壻
��6��������ͭ�����ڰ�ˮ�γ��İ���ͭ�����ӣ�
��7�����ݾ�̯�����㾧����ԭ����Ŀ��
��� �⣺����Ԫ�������ڱ��е�λ�ÿ�֪��aΪHԪ�ء�bΪBeԪ�ء�cΪCԪ�ء�dΪNԪ�ء�eΪOԪ�ء�fΪFԪ�ء�gΪNaԪ�ء�hΪPԪ�ء�iΪSԪ�ء�jΪCuԪ�أ�
��1��jΪCuԪ�أ�ԭ�Ӻ��������ĿΪ29����������Ų�ʽΪ1s22s22p63s23p63d104s1��aΪHԪ�ء�cΪCԪ�أ���ca4����ΪCH4��Ԫ��CΪsp3�ӻ������ӵĿռ乹��Ϊ�������壬�ʴ�Ϊ��1s22s22p63s23p63d104s1��sp3���������壻
��2��ci2����ΪCS2����ԭ����̼ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ��ce2�ķ���ʽCO2����CS2�Ƚϣ�����̼���۷е�ߣ���Ϊ�����ӽṹ���ƣ�Ϊ���Ӿ��壬���Ӿ�����۷е�ߵ�ȡ������Է��������Ĵ�С��
��ce2�ķ���ʽCO2����CS2�Ƚϣ�����̼���۷е�ߣ���Ϊ�����ӽṹ���ƣ�Ϊ���Ӿ��壬���Ӿ�����۷е�ߵ�ȡ������Է��������Ĵ�С��
�ʴ�Ϊ�� ��CS2��
��CS2��
��3��da3��ha3��ȣ�NH3�ķе�Ҫ�ߵö�ԭ��ΪNH3���Ӽ��γ�������ʴ�Ϊ��NH3���Ӽ��γ������
��4��PԪ�ص�3p���Ϊ�����ȶ�״̬���������ͣ��ʵ�һ������P��S��������Na��Be��������Խǿ���縺��ԽС���ʵ縺��Na��Be���ʴ�Ϊ����������
��5��ce2����ΪCO2����ȵ����������ΪCS2�ȣ��ʴ�Ϊ��CS2��
��6��������ͭ�����ڰ�ˮ�γ�[Cu��NH3��4]2+�����ӷ���ʽΪ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
�ʴ�Ϊ��[Cu��NH3��4]2+��
��7����Cu������֪�������к���Cuԭ����ĿΪ6��$\frac{1}{2}$+8��$\frac{1}{8}$=4��
�ʴ�Ϊ��4��
���� �����Ƕ����ʽṹ�������ۺϿ��飬֪ʶ�㿼��ȫ�棬�ѶȲ�������֪ʶ������У�ע�����֪ʶ��ȫ�����գ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | 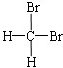 �� �� 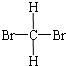 | B�� | 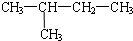 �� ��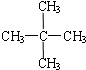 | C�� | CH4��CH3CH3 | D�� | ���ۺ���ά�� |
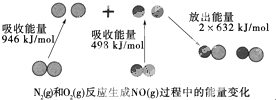 ��ѧ��Ӧ�е������仯���ɻ�ѧ��Ӧ�оɻ�ѧ������ʱ���յ��������»�ѧ���γ�ʱ�ų���������ͬ����ģ���ͼΪN2��g����O��g����Ӧ����NO��g�������е������仯������˵������ȷ���ǣ�������
��ѧ��Ӧ�е������仯���ɻ�ѧ��Ӧ�оɻ�ѧ������ʱ���յ��������»�ѧ���γ�ʱ�ų���������ͬ����ģ���ͼΪN2��g����O��g����Ӧ����NO��g�������е������仯������˵������ȷ���ǣ�������| A�� | 1 mol N2��g����1 mol O2��g����Ӧ�ų�������Ϊ180 kJ | |
| B�� | 1 mol N2��g����1 mol O2��g�������������2 mol NO��g����������� | |
| C�� | ͨ������£�N2��g����O2��g�������ֱ������NO | |
| D�� | NO��һ���������������NaOH��Һ��Ӧ�����κ�ˮ |
�ٹ������ȶ��ԣ�Na2CO3��CaCO3��NaHCO3
�����뾶��F-��Na+��Mg2+��S2-
��ȼ���ȣ�S�����壩��S��Һ�壩��S�����壩
�����ʵ��۵㣺ֲ���ͣ������ͣ�
| A�� | �٢ۢ� | B�� | �٢� | C�� | �٢ڢۢ� | D�� | �ڢۢ� |
���ȼ���$\stackrel{HF��SbCl_{3}}{��}$����һ�ȼ���$\stackrel{��}{��}$�ķ���ϩ$\stackrel{������}{��}$���ķ���ϩ
����˵���У�����ȷ���ǣ�������
| A�� | ����һ�ȼ�Ժ ��CHClF2��������ԭ������㶼�ﵽ��8�����ȶ��ṹ | |
| B�� | ��������������ʹ���Ը��������Һ��ɫ | |
| C�� | �ķ���ϩ ��CF2=CF2�������е�ԭ�Ӷ���ͬһ��ƽ���� | |
| D�� | ���ȼ��飨CHCl3�������ü�Ժ��������ȡ����Ӧ����ȡ |
| A�� | B�� |
| ��H-I�����ܴ���H-Cl������ ��H-I������С��H-Cl������ ��HI���Ӽ�����������HCl���Ӽ������� ����HI���Ӽ�������С��HCl���Ӽ������� | ��HI��HCl�ȶ� ��HI��HCl���ȶ� ��HI�е��HCl�� ��HI�е��HCl�� |
| A�� | �� | B�� | ������ | C�� | �� | D�� | �� |
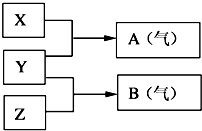 ��A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��W ͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȣ�
��A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��W ͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȣ�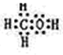 ��
��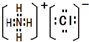 ��
��