题目内容
决定物质性质的重要因素是物质结构,请回答下列问题。
(1)已知A和B为第三周期元素,其原子的第一至第四电离能如下表所示:
A通常显 价,A的电负性 B的电负性(填“>”、“<”或“=”)。
(2)紫外光的光子所具有的能量约为399 kJ·mol-1。根据下表有关蛋白质分子中重要化学键的信息,说明人体长时间照射紫外光后皮肤易受伤害的原因 。
组成蛋白质的最简单的氨基酸中的碳原子杂化类型是 。
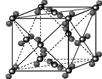
(3)实验证明:KCl、MgO、CaO、TiN这4种晶体的结构与NaCl晶体结构相似(如图所示),已知3种离子晶体的晶格能数据如下表:

则该4种离子晶体(不包括NaCl)熔点从高到低的顺序是: 。其中MgO晶体中一个Mg2+周围和它最邻近且等距离的Mg2+有 个。
(4)金属阳离子含未成对电子越多,则磁性越大,磁记录性能越好。离子型氧化物V2O5和CrO2中,适合作录音带磁粉原料的是 。
(5)某配合物的分子结构如图所示,其分子内不含有 (填序号)。

A离子键;B极性键;C金属键;D配位键;E氢键;F非极性键
(1)已知A和B为第三周期元素,其原子的第一至第四电离能如下表所示:
| 电离能/kJ·mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1 817 | 2 745 | 11 578 |
| B | 738 | 1 451 | 7 733 | 10 540 |
A通常显 价,A的电负性 B的电负性(填“>”、“<”或“=”)。
(2)紫外光的光子所具有的能量约为399 kJ·mol-1。根据下表有关蛋白质分子中重要化学键的信息,说明人体长时间照射紫外光后皮肤易受伤害的原因 。
组成蛋白质的最简单的氨基酸中的碳原子杂化类型是 。
| 共价键 | C—C | C—N | C—S |
| 键能/kJ·mol-1 | 347 | 305 | 259 |
| 离子晶体 | NaCl | KCl | CaO |
| 晶格能/kJ·mol-1 | 786 | 715 | 3 401 |

则该4种离子晶体(不包括NaCl)熔点从高到低的顺序是: 。其中MgO晶体中一个Mg2+周围和它最邻近且等距离的Mg2+有 个。
(4)金属阳离子含未成对电子越多,则磁性越大,磁记录性能越好。离子型氧化物V2O5和CrO2中,适合作录音带磁粉原料的是 。
(5)某配合物的分子结构如图所示,其分子内不含有 (填序号)。

A离子键;B极性键;C金属键;D配位键;E氢键;F非极性键
(1)+3 >
(2)紫外光具有的能量比蛋白质分子中主要化学键C—C、C—N和C—S的键能都大,紫外光的能量足以使这些化学键断裂,从而破坏蛋白质分子 sp2和sp3
(3)TiN>MgO>CaO>KCl 12
(4)CrO2
(5)C
(2)紫外光具有的能量比蛋白质分子中主要化学键C—C、C—N和C—S的键能都大,紫外光的能量足以使这些化学键断裂,从而破坏蛋白质分子 sp2和sp3
(3)TiN>MgO>CaO>KCl 12
(4)CrO2
(5)C
(1)根据A的第4电离能突然增大很多,A通常显示+3价;根据B的第3电离能突然增大,可判断B通常显示+2价,故在周期表中,A在B的右边,故A的电负性大于B。
(2)根据表中数据可知紫外光光子所具有的能量大于蛋白质中各种化学键的键能,因此紫外光会破坏蛋白质中化学键进而破坏蛋白质。
(3)离子晶体晶格能越大,熔沸点越高。而晶格能与阴阳离子所带的电荷的乘积成正比,与阴阳离子之间的距离成反比,且电荷的影响更大,TN中阴阳离子各带3个单位电荷,CaO中各带2个单位电荷,MgO中各带2个单位电荷,KCl中各带1个单位电荷,故熔沸点高低为:TN>MgO>CaO>KCl。如果中心黑点代表Mg2+,则到每条棱的中点最近,故有12个。
(4)V的价电子排布为3d34s2,故V5+没有未成对电子,而Cr的价电子排布为3d54s1,故Cr4+有2个未成对的电子,故CrO2作为录音带磁粉原料。
(5)根据图示可看出,O为阴离子,带一个单位负电荷,故含离子键;N与Ni之间存在配位键,O和H之间的虚线为氢键,N和C、C和H等之间是极性共价键,C和C之间为非极性共价键。
(2)根据表中数据可知紫外光光子所具有的能量大于蛋白质中各种化学键的键能,因此紫外光会破坏蛋白质中化学键进而破坏蛋白质。
(3)离子晶体晶格能越大,熔沸点越高。而晶格能与阴阳离子所带的电荷的乘积成正比,与阴阳离子之间的距离成反比,且电荷的影响更大,TN中阴阳离子各带3个单位电荷,CaO中各带2个单位电荷,MgO中各带2个单位电荷,KCl中各带1个单位电荷,故熔沸点高低为:TN>MgO>CaO>KCl。如果中心黑点代表Mg2+,则到每条棱的中点最近,故有12个。
(4)V的价电子排布为3d34s2,故V5+没有未成对电子,而Cr的价电子排布为3d54s1,故Cr4+有2个未成对的电子,故CrO2作为录音带磁粉原料。
(5)根据图示可看出,O为阴离子,带一个单位负电荷,故含离子键;N与Ni之间存在配位键,O和H之间的虚线为氢键,N和C、C和H等之间是极性共价键,C和C之间为非极性共价键。

练习册系列答案
相关题目











 与X、Y形成的一种化合物互为等电子体,则
与X、Y形成的一种化合物互为等电子体,则