��Ŀ����
����һ����Ҫ�Ļ�����Ʒ��Ҳ�ǻ�����������Ҫԭ�ϣ�
��1��ʵ����ͨ�������ֹ��������Ʊ�������д���˷�Ӧ�Ļ�ѧ����ʽ �����������������ԭ���� ��
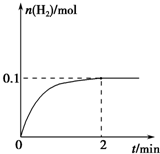
��2����������25mL 0.01mol?L-1ϡ�����л���ͨ��5.6mL NH3����״���£���Һ����仯���Բ��ƣ�����ͨ��NH3�Ĺ�������Һ�ĵ������� ����������С���������䡱����
��3����������Һ�м���ͨ��NH3���ù���������Ũ�ȴ�С��ϵ������ȷ���� ������ţ���
A��c��Cl-��=c��NH4+����c��H+��=c��OH-��
B��c��Cl-����c��NH4+��=c��H+����c��OH-��
C��c��NH4+����c��OH-����c��Cl-����c��H+��
D��c��OH-����c��NH4+����c��H+����c��Cl-��
��4����������25mL��0.01mol HCl����Һ�еμ�25mL��ˮ�������Һ��ˮ�ĵ���������ˮ��Ũ��Ϊ mol?L-1��
��1��ʵ����ͨ�������ֹ��������Ʊ�������д���˷�Ӧ�Ļ�ѧ����ʽ
��2����������25mL 0.01mol?L-1ϡ�����л���ͨ��5.6mL NH3����״���£���Һ����仯���Բ��ƣ�����ͨ��NH3�Ĺ�������Һ�ĵ�������
��3����������Һ�м���ͨ��NH3���ù���������Ũ�ȴ�С��ϵ������ȷ����
A��c��Cl-��=c��NH4+����c��H+��=c��OH-��
B��c��Cl-����c��NH4+��=c��H+����c��OH-��
C��c��NH4+����c��OH-����c��Cl-����c��H+��
D��c��OH-����c��NH4+����c��H+����c��Cl-��
��4����������25mL��0.01mol HCl����Һ�еμ�25mL��ˮ�������Һ��ˮ�ĵ���������ˮ��Ũ��Ϊ
���㣺����Ũ�ȴ�С�ıȽ�,���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
��������1��ʵ�������Ȼ�狀��������Ƽ�����ȡ������������Һ��������ʱ����������
��2��n��HCl��=0.01mol��0.025L=2.5��10-4 mol�����������ʵ���=
=2.5��10-4 mol������ǡ�÷�Ӧ�����Ȼ�泥���Һ������Ũ�ȼ������䣻
��3����������Һ�м���ͨ��NH3����Һ���ܳʼ��Ի����ԣ�
��4���������ˮ���룬������ǡ�÷�Ӧ�����Ȼ��ʱ��ˮ�ĵ���̶����
��2��n��HCl��=0.01mol��0.025L=2.5��10-4 mol�����������ʵ���=
| 5.6��10-3L |
| 22.4L/mol |
��3����������Һ�м���ͨ��NH3����Һ���ܳʼ��Ի����ԣ�
��4���������ˮ���룬������ǡ�÷�Ӧ�����Ȼ��ʱ��ˮ�ĵ���̶����
���
�⣺��1��ʵ�������Ȼ�狀��������Ƽ�����ȡ��������Ӧ����ʽΪCa��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O��������Һ��������ʱ����������������Χ�¶Ƚ��ͣ����������������
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O������Һ����Һ������ʱ���մ������ȣ�
��2��n��HCl��=0.01mol��0.025L=2.5��10-4 mol�����������ʵ���=
=2.5��10-4 mol������ǡ�÷�Ӧ�����Ȼ�泥���Һ������Ũ�ȼ������䣬���Ե��������������䣬�ʴ�Ϊ���������䣻
��3��A����Һ������ʱ��c��H+��=c��OH-�������ݵ���غ��c��Cl-��=c��NH4+������Һ��ˮ�ĵ���̶Ⱥ�С�����Դ���c��Cl-��=c��NH4+����c��H+��=c��OH-������A��ȷ��
B������ͨ�백��ʱ��ˮ�ĵ���̶ȼ�С�����ܳ���c��NH4+��=c��H+������B����
C����Һ�е�����Ϊһˮ�ϰ����Ȼ����һˮ�ϰ�Ũ��ԶԶ�����Ȼ��ʱ�����ܴ���c��NH4+����c��OH-����c��Cl-����c��H+������C��ȷ��
D����Һ�ʼ���ʱ����Һ��������Ũ�Ⱥ�С�����ܳ���c��H+����c��Cl-������D����
��ѡA C��
��4���������ˮ���룬������ǡ�÷�Ӧ�����Ȼ��ʱ��ˮ�ĵ���̶������һˮ�ϰ������ʵ���Ϊ0.01mol����Ũ��=
=0.4mol/L��
�ʴ�Ϊ��0.4��
| ||
�ʴ�Ϊ��Ca��OH��2+2NH4Cl
| ||
��2��n��HCl��=0.01mol��0.025L=2.5��10-4 mol�����������ʵ���=
| 5.6��10-3L |
| 22.4L/mol |
��3��A����Һ������ʱ��c��H+��=c��OH-�������ݵ���غ��c��Cl-��=c��NH4+������Һ��ˮ�ĵ���̶Ⱥ�С�����Դ���c��Cl-��=c��NH4+����c��H+��=c��OH-������A��ȷ��
B������ͨ�백��ʱ��ˮ�ĵ���̶ȼ�С�����ܳ���c��NH4+��=c��H+������B����
C����Һ�е�����Ϊһˮ�ϰ����Ȼ����һˮ�ϰ�Ũ��ԶԶ�����Ȼ��ʱ�����ܴ���c��NH4+����c��OH-����c��Cl-����c��H+������C��ȷ��
D����Һ�ʼ���ʱ����Һ��������Ũ�Ⱥ�С�����ܳ���c��H+����c��Cl-������D����
��ѡA C��
��4���������ˮ���룬������ǡ�÷�Ӧ�����Ȼ��ʱ��ˮ�ĵ���̶������һˮ�ϰ������ʵ���Ϊ0.01mol����Ũ��=
| 0.01mol |
| 0.025L |
�ʴ�Ϊ��0.4��
���������⿼��������Ũ�ȴ�С�Ƚϣ�������Һ�е����ʼ���Һ������ٽ���غ�˼����������ȷ��4�����ʱˮ�ĵ���̶����Ϊ�״��㣮

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
��ͼ�ǵ����ҺCuCl2��װ�ã�����c��dΪʯī�缫���������й��ж���ȷ���ǣ�������


| A��a������d���� |
| B��c�缫������ԭ��Ӧ |
| C���������У�d�缫�������� |
| D���������У�������Ũ�Ȳ��� |
���з�����ȷ���ǣ�������
A��[SiO4]��������ṹ�� |
| B��C2H5Cl��NaOH��Һ��ϼ���ˮ�����AgNO3��Һ���Լ���Cl- |
| C����MgCl2�����к��й��ۼ������Ӽ� |
| D��0.5g C3H4�к��й��õ��ӶԵ���ĿΪ1��6.02��1023 |
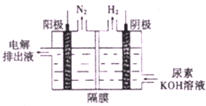 ������һ�������Դ����������ȡ������������Դ������о��ȵ�
������һ�������Դ����������ȡ������������Դ������о��ȵ�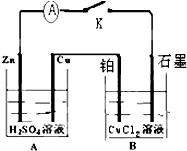 ��ͼ���ش����⣺
��ͼ���ش����⣺

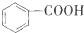 ��-R1��-R2��ʾ��ԭ�ӻ���������
��-R1��-R2��ʾ��ԭ�ӻ���������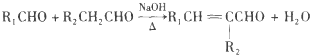
 ��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��