��Ŀ����
18�� ���ۼ����м���֮˵��������ָ��1mol���ۼ�����Ҫ���յ��������γ�1mol���ۼ����ų���������
���ۼ����м���֮˵��������ָ��1mol���ۼ�����Ҫ���յ��������γ�1mol���ۼ����ų�����������1����֪H-Cl���ļ���Ϊ431.4kJ/mol�����й��ڼ��ܵ�������ȷ����AD��
A��ÿ����1mol H-Cl���ų�431.4kJ���� B��ÿ����1mol H-Cl������431.4kJ����
C��ÿ��1mol H-Cl���ų�431.4kJ���� D��ÿ��1mol H-Cl������431.4kJ����
��2���ο��±��е����ݣ��ж����з�������ʱ���ȶ�����A��
| ��ѧ�� | H-H | H-F | H-Cl | H-Br |
| ����/kJ/mol | 436 | 565 | 431 | 368 |
��3�����ü��ܴ�С���͵���A��
A�������Ļ�ѧ���ʱ������ȶ�
B�����³�ѹ�£����Һ̬����ʹ�̬
C��ϡ������һ����ѷ�����ѧ��Ӧ
D�������ӷ��������ѻӷ�
��4����֪��4HCl+O2�T2Cl2+2H2O���÷�Ӧ�У�4mol HCl���������ų�115.6kJ���������Ͽ�1mol H-O����Ͽ�1mol H-Cl�������������ԼΪ31.9kJ��
���� ��1�����ݼ��ܵĺ����֪����ѧ��Ҫ�����������γɻ�ѧ���ų���������֪H-Cl���ļ���Ϊ431.4kJ/mol�����ݼ��ܸ��������
��2������Խ��Խ�ȶ���
��3��A������Խ����Խ�ȶ���
B�����Ӽ�������Խ���Ӿ�����۵�Խ�ߣ�
C��ϡ������Ϊ��ԭ�ӷ��ӣ�û�л�ѧ����
D��������ӷ�������е��йأ�
��4����Ӧ�ȡ�H=��Ӧ���ܼ���-��������ܼ��ܣ��ݴ˼���H-O����H-Cl���ļ��ܲ��������Ͽ�1mol H-O����Ͽ�1mol H-Cl�����������
��� �⣺��1����֪H-Cl���ļ���Ϊ431.4kJ/mol������Ҫ��1 molH-Cl����Ҫ����431.4kJ������Ҫ�γ�1 molH-Cl����Ҫ�ų�431.4kJ������
�ʴ�Ϊ��AD��
��2������Խ��Խ�ȶ����ɱ������ݿ�֪��HF�ļ��������HF���ȶ����ʴ�Ϊ��A��
��3��A������Խ����Խ�ȶ��������еĹ��ۼ��ļ��ܱ������Ĵ����Ե����Ļ�ѧ���ʱ������ȶ������ü��ܽ��ͣ���A��ȷ��
B�����Ӽ�������Խ���Ӿ�����۵�Խ�ߣ����³�ѹ�£����Һ̬����ʹ�̬������Ϊ���ʵ�ķ��Ӽ���������������أ���B����
C��ϡ������Ϊ��ԭ�ӷ��ӣ�û�л�ѧ�������ѷ�����ѧ��Ӧ������Ϊԭ�Ӵﵽ8�����ȶ��ṹ�������γɻ�ѧ������C����
D��������ӷ�������е��йأ����Ӿ���ķе�����Ӽ��������йأ�������أ���D����
�ʴ�Ϊ��A��
��4��E��H-O����E��HCl���ֱ��ʾH-O���ܡ�H-Cl���ܣ���ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ�ȡ�H=��Ӧ���ܼ���-��������ܼ��ܣ��ʣ�
4��E��H-Cl��+498kJ/mol-[2��243kJ/mol+4��E��H-O��]=-116kJ/mol��
�����ã�4E��H-Cl��-4E��H-O��=-127.6kJ/mol����E��H-O��-E��HCl��=31.9kJ/mol��
�ʶϿ�1mol H-O����Ͽ�1mol H-Cl�������������ԼΪ32kJ/mol��1mol=31.9kJ��
�ʴ�Ϊ��31.9��
���� ���⿼���˼�������Ӽ������������𡢷�Ӧ�ȵļ��㣬����ʱ��������Ŀ�������ϵ���������Ӽ�������������״̬�йأ�������أ���Ŀ�Ѷ��еȣ�

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�| A�� | �����£�7.8g����Na2O2�У����е�������������Ϊ0.4NA | |
| B�� | 4��ʱ��18g 2H216O�к��й��õ��Ӷ���Ϊ2NA | |
| C�� | 1 mol N5+���еĵ�����Ϊ34NA | |
| D�� | �ý�������CuƬ��ϡ�������ԭ��أ�����������������5.6gʱ���������·�ĵ���Ϊ0.3NA |
| A�� | ����ȼ�� | B�� | Ba��OH��2•8H2O��NH4Cl��Ӧ | ||
| C�� | ��Ƭ�����ᷴӦ | D�� | ��������ˮ��Ӧ |
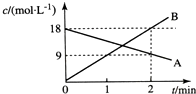
| A�� | v��A��=9 mol/��L•min�� | B�� | v��B��=18 mol/��L•min�� | ||
| C�� | v��A��=4.5 mol/��L•min�� | D�� | v��B��=4.5 mol/��L•min�� |
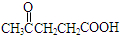 ��CΪHOOC-COOH��A�ĺ������ͼ�ͺ˴Ź�������ͼ��ͼ1��
��CΪHOOC-COOH��A�ĺ������ͼ�ͺ˴Ź�������ͼ��ͼ1��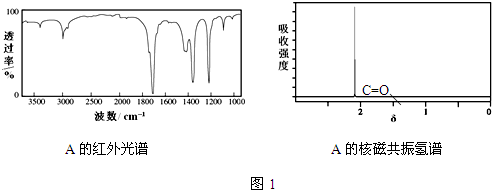

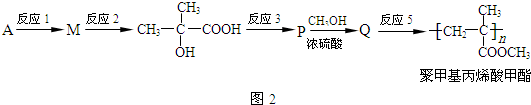
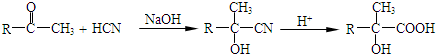
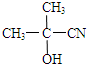 ��
��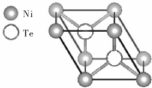 ����O������S��������Se�����ڣ�Te��Ϊ��A��Ԫ�أ���ش��������⣺
����O������S��������Se�����ڣ�Te��Ϊ��A��Ԫ�أ���ش��������⣺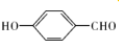 �ķе��
�ķе�� �ߣ�ԭ���Ƕ��ǻ�����ȩ���Ӽ����γ���������ǻ�����ȩ���������γ������
�ߣ�ԭ���Ƕ��ǻ�����ȩ���Ӽ����γ���������ǻ�����ȩ���������γ������