��Ŀ����
����Ŀ�������£�������Һ�е�����Ũ�ȹ�ϵ��ȷ���ǣ� ��
A. һ�����ʵ���Ũ�ȵ�Na2S��Һ�У�c(OH��)��c(H��)��2c(H2S)��c(HS��)
B. ������ˮ�м������NaOH��c(Na��)��c(Cl��)��c(ClO��)��c(OH��)
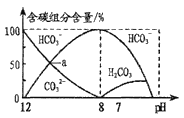
C. pH��8.3��NaHCO3��Һ��c(Na��)>c(HCO3-)>c(CO32-)>c(H2CO3)
D. 25��ʱ��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�����ҺpH��4.75��c(![]() )+c(
)+c(![]() )=c(CH3COOH)+c(H+)
)=c(CH3COOH)+c(H+)
���𰸡�A
��������A����Na2S��Һ�������������غ��c��OH����=c��H+��+2c��H2S��+c��HS��������A��ȷ��B��������ˮ�м������NaOH�����ݵ���غ��c��Na+��+c��H+��=c��Cl����+c��ClO����+c��OH����������c��Na+����c��Cl����+c��ClO����+c��OH��������B����C��pH=8.3��NaHCO3��Һ����Һ�ʼ��ԣ�˵��HCO3����ˮ��̶ȴ��ڵ���̶ȣ�����c��CO32������c��H2CO3������C����D��25��ʱ��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COOH��CH3COONa�Ļ����Һ�д��ڵ���غ���c��CH3COO����+c��OH����=c��Na+��+c��H+����25��ʱ����ҺpH=4.75������Һ�д������̶ȴ��ڴ��������ˮ��̶ȣ�����c��Na+����c��CH3COOH������c��CH3COO����+c��OH������c��CH3COOH��+c��H+������D����ѡA��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�