��Ŀ����
����Ŀ��CoS2��CO����������й����Ĺ�ҵ��ǰ�����ش��������⣺
(1)��֪��
CoS2(s) +CO(g) ![]() CoS(s) +COS(g) H1
CoS(s) +COS(g) H1
2COS(g) +SO2(g) ![]() 3S(s) +2CO2(g) H2
3S(s) +2CO2(g) H2
S(s) +CoS(S) ![]() CoS2 (s) ��H3
CoS2 (s) ��H3
��2CO(g)+ SO2(g)![]() 2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
2CO2(g)+S(s) H4=____�� (��H1�� H2��H3��ʾ)
(2)�ں��¡���ѹ��������ģ���������SO2��ʼ����Ϊ1mol�����CO2��ƽ�����������CO��SO2��Ͷ�ϱȱ仯��ͼ��

�ٵ�Ͷ�ϱ�Ϊ2ʱ��t min ʱ���SO2ת����Ϊ50%������S���������ʱ�ʾ�ķ�Ӧ����v=______g��min-1��
�ڵ�Ͷ�ϱ�Ϊ3ʱ��CO2 ��ƽ�����������Ӧ�ĵ���______________��
(3)�������Ϊ1L�ĺ��¡������������ͨ��2 mol CO��| mol SO2����Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��
�������I��II�ı�����������____________________��
��SO2��ƽ��ת����Ϊ______��ƽ�ⳣ��Kp =________(��ƽ���ѹ����ƽ��Ũ�ȼ���)��
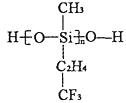
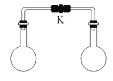
(4)���õ�ⷨ����SO2β�����Ʊ����շ� (Na2S2O4).���װ����ͼ����a____ b (����>�� ��=������<��)������S2O42-�ĵ缫��ӦʽΪ____________________��

���𰸡�![]()
![]() C ʹ�ã���ʹ�ø���Ч������ 75% 0.675 <
C ʹ�ã���ʹ�ø���Ч������ 75% 0.675 < ![]()
��������
��1�����ݸ�˹���ɿ�֪![]() �ɵ������Ȼ�ѧ����ʽ��
�ɵ������Ȼ�ѧ����ʽ��
��2�����������η�����S����������S����������![]() ���㣻�ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2�����������
���㣻�ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2�����������
��3������ͼ���֪I��II���ﵽƽ��ʱѹǿ���䣬��ƽ�ⲻ�ƶ���II�ﵽƽ��ʱ���̣���IIʹ�ã���ʹ�ø���Ч���������ں��¡�������������������ѹǿ�����ʵ��������ȣ��������η�����SO2��ƽ��ת���ʣ�ƽ�ⳣ��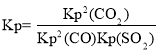 ���Դ˼��㣻
���Դ˼��㣻
��4���ɵ��װ��ͼ��֪��SO2����������Ӧ����H2SO4��Ϊ�������缫��ӦΪ��![]() ��HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��
��HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��![]() ���Դ˷�����
���Դ˷�����
��1��![]()
![]()
![]() ���ݸ�˹���ɿ�֪
���ݸ�˹���ɿ�֪![]() �ɵ�
�ɵ�![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2���ٵ�Ͷ�ϱ�Ϊ2ʱ��t min ʱ���SO2ת����Ϊ50%������
S����������![]() ���ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2������������ʴ�Ϊ��
���ڵ�Ͷ�ϱ�Ϊ3ʱ���൱����Ͷ�ϱ�Ϊ2�ﵽƽ��ʱ����1mol��CO��ƽ�����ƣ�������������ԭ����֪�ﵽƽ��ʱ��CO2���������С��Ͷ�ϱ�Ϊ2�ﵽƽ��ʱCO2������������ʴ�Ϊ��![]() ��C��
��C��
��3������ͼ���֪I��II���ﵽƽ��ʱѹǿ���䣬��ƽ�ⲻ�ƶ���II�ﵽƽ��ʱ���̣���IIʹ�ã���ʹ�ø���Ч���������ں��¡�������������������ѹǿ�����ʵ��������ȣ��跴Ӧ����SO2���ʵ���Ϊxmol������
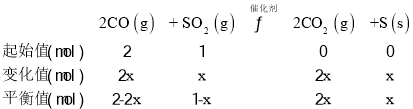
![]() ��x=0.75mol��SO2��ƽ��ת����Ϊ
��x=0.75mol��SO2��ƽ��ת����Ϊ![]() ����ƽ��ʱn��CO��=0.5mol��n��SO2��=0.25mol��n��CO2��=1.5mol����ƽ�ⳣ��
����ƽ��ʱn��CO��=0.5mol��n��SO2��=0.25mol��n��CO2��=1.5mol����ƽ�ⳣ��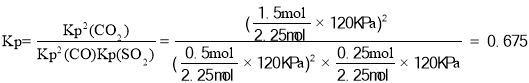 ���ʴ�Ϊ��ʹ�ã���ʹ�ø���Ч��������75%��0.675��
���ʴ�Ϊ��ʹ�ã���ʹ�ø���Ч��������75%��0.675��
��4���ɵ��װ��ͼ��֪��SO2����������Ӧ����H2SO4��Ϊ�������缫��ӦΪ��![]() ��������Ũ������HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��
��������Ũ������HSO3-������ԭ��Ӧ����S2O42-��Ϊ�������缫��ӦΪ��![]() ���ʴ�Ϊ��<��
���ʴ�Ϊ��<��![]() ��
��