ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ÷–ΩΤ‘Κ¥σΝ§Μ·―ßΈοάμ―–ΨΩΥυΒΡ“ΜœνΉν–¬≥…Ιϊ Βœ÷ΝΥΦΉΆιΗΏ–ß…ζ≤ζ““œ©Θ§»γΆΦΥυ ΨΘ§ΦΉΆι‘Ύ¥ΏΜ·Ής”Οœ¬Ά―«βΘ§‘Ύ≤ΜΆ§Έ¬Ε»œ¬Ζ÷±π–Έ≥…![]() Β»Ή‘”…ΜυΘ§‘ΎΤχœύ÷–Ψ≠Ή‘”…ΜυΘΚCH2≈ΦΝΣΖ¥”Π…ζ≥…““œ©(ΗΟΖ¥”ΠΙΐ≥ΧΩ…Ρφ)
Β»Ή‘”…ΜυΘ§‘ΎΤχœύ÷–Ψ≠Ή‘”…ΜυΘΚCH2≈ΦΝΣΖ¥”Π…ζ≥…““œ©(ΗΟΖ¥”ΠΙΐ≥ΧΩ…Ρφ)

Έο÷ | »Φ…’»»/(kJmol-1) |
«βΤχ | 285.8 |
ΦΉΆι | 890.3 |
““œ© | 1411.0 |
(1)“―÷ΣœύΙΊΈο÷ ΒΡ»Φ…’»»»γ…œ±μΥυ ΨΘ§–¥≥ωΦΉΆι÷Τ±Η““œ©ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ______________ΓΘ
(2)œ÷¥ζ ·”ΆΜ·ΙΛ≤…”ΟAgΉς¥ΏΜ·ΦΝΘ§Ω… Βœ÷““œ©”κ―θΤχ÷Τ±ΗX(Ζ÷Ή” ΫΈΣC2H4OΘ§≤ΜΚ§ΥΪΦϋ)ΗΟΖ¥”ΠΖϊΚœΉνάμœκΒΡ‘≠Ή”Ψ≠ΦΟΘ§‘ρΖ¥”Π≤ζΈο «____________(ΧνΫαΙΙΦρ Ϋ)
(3)‘Ύ400Γφ ±Θ§œρ≥θ ΦΧεΜΐΈΣ1LΒΡΚψ―ΙΟή±’Ζ¥”ΠΘ§Τς÷–≥δ»κ1 mol CH4Θ§ΖΔ…ζ(1)÷–Ζ¥”ΠΘ§≤βΒΟΤΫΚβΜλΚœΤχΧε÷–C2H4ΒΡΧεΜΐΖ÷ ΐΈΣ25.0%ΓΘ‘ρΘΚ

ΔΌ‘ΎΗΟΈ¬Ε»œ¬Θ§ΤδΤΫΚβ≥Θ ΐKCΘΫ____________ΓΘ
ΔΎ»τœρΗΟΖ¥”ΠΤς÷–Ά®»κΗΏΈ¬Υ°’τΤχ(≤Μ≤ΈΦ”Ζ¥”ΠΘ§ΗΏ”Ύ400Γφ)Θ§‘ρC2H4ΒΡ≤ζ¬ ____________ΓΘ(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΓΑ≤Μ±δΓ±ΜρΓΑΈόΖ®»ΖΕ®Γ±)Θ§άμ”… «_______________________________ΓΘ
Δέ»τΖ¥”ΠΤςΒΡΧεΜΐΙΧΕ®Θ§≤ΜΆ§―Ι«Ωœ¬Ω…ΒΟ±δΜ·»γ”“ΆΦΥυ ΨΘ§‘ρ―Ι«ΩP1Θ§P2ΒΡ¥σ–ΓΙΊœΒ «____________ΓΘ

(4) ΒΦ ÷Τ±ΗC2H4 ±Θ§Ά®≥Θ¥φ‘ΎΗ±Ζ¥”Π2CH4(g)![]() C2H4(g)ΘΪ2H2(g)ΓΘΖ¥”ΠΤςΚΆCH4Τπ ΦΝΩ≤Μ±δΘ§≤ΜΆ§Έ¬Ε»œ¬C2H6ΚΆC2H4ΒΡΧεΜΐΖ÷ ΐ”κΈ¬Ε»ΒΡΙΊœΒ«ζœΏ»γ”“ΆΦΥυ ΨΓΘ‘ΎΈ¬Ε»ΗΏ”Ύ600Γφ ±Θ§”–Ω…ΡήΒΟΒΫ“Μ÷÷ΫœΕύΒΡΥΪΧΦ”–ΜζΗ±≤ζΈοΒΡΟϊ≥Τ «____________ΓΘ
C2H4(g)ΘΪ2H2(g)ΓΘΖ¥”ΠΤςΚΆCH4Τπ ΦΝΩ≤Μ±δΘ§≤ΜΆ§Έ¬Ε»œ¬C2H6ΚΆC2H4ΒΡΧεΜΐΖ÷ ΐ”κΈ¬Ε»ΒΡΙΊœΒ«ζœΏ»γ”“ΆΦΥυ ΨΓΘ‘ΎΈ¬Ε»ΗΏ”Ύ600Γφ ±Θ§”–Ω…ΡήΒΟΒΫ“Μ÷÷ΫœΕύΒΡΥΪΧΦ”–ΜζΗ±≤ζΈοΒΡΟϊ≥Τ «____________ΓΘ
(5)C2H4ΓΔC2H6≥Θ≥ΘΉςΈΣ»ΦΝœΒγ≥ΊΒΡ‘≠ΝœΘ§«κ–¥≥ωC2H4‘ΎNaOH»ή“Κ÷–Ήω»ΦΝœΒγ≥ΊΒΡΗΚΦΪΒΡΒγΦΪΖ¥”ΠΖΫ≥Χ Ϋ________________________________________________ΓΘ
ΓΨ¥πΑΗΓΩ2CH4(g)![]() C2H4(g)+2H2(g) ΠΛH=+202.0kJ/mol)
C2H4(g)+2H2(g) ΠΛH=+202.0kJ/mol) ![]() 1.0 ‘ω¥σ ΗΟΖ¥”ΠΈΣΤχΧεΧεΜΐ‘ω¥σΒΡΈϋ»»Ζ¥”ΠΘ§Ά®»κΗΏΈ¬Υ°’τΤϊœύΒ±”ΎΦ”»»Θ§Ά§ ±Ά®»κΥ°’τΤχΘ§Ζ¥”ΠΤςΒΡΧεΜΐ‘ω¥σΘ§œύΒ±”ΎΦθ–Γ―Ι«ΩΘ§Ψυ ΙΤΫΚβ”““ΤΘ§C2H4ΒΡ≤ζ¬ ‘ω¥σ P1>P2 ““»≤ 16OH-+C2H4-12e-=2CO32-+10H2O
1.0 ‘ω¥σ ΗΟΖ¥”ΠΈΣΤχΧεΧεΜΐ‘ω¥σΒΡΈϋ»»Ζ¥”ΠΘ§Ά®»κΗΏΈ¬Υ°’τΤϊœύΒ±”ΎΦ”»»Θ§Ά§ ±Ά®»κΥ°’τΤχΘ§Ζ¥”ΠΤςΒΡΧεΜΐ‘ω¥σΘ§œύΒ±”ΎΦθ–Γ―Ι«ΩΘ§Ψυ ΙΤΫΚβ”““ΤΘ§C2H4ΒΡ≤ζ¬ ‘ω¥σ P1>P2 ““»≤ 16OH-+C2H4-12e-=2CO32-+10H2O
ΓΨΫβΈωΓΩ
(1)ΗυΨί±μΗώ÷– ΐΨί”–ΘΚΔΌH2(g)+![]() O2(g)®TH2O(l)ΓςH1=-285.8kJ/molΘΜ
O2(g)®TH2O(l)ΓςH1=-285.8kJ/molΘΜ
ΔΎCH4(g)+2O2(g)=CO2(g)+2H2O(l)ΓςH2=-890.3kJ/molΘΜ
ΔέC2H4(g)+3O2(g)=2CO2(g)+2H2O(l)ΓςH3=-1411.0kJ/molΘΜ
ΦΉΆι÷Τ±Η““œ©ΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ2CH4(g)C2H4(g)+2H2(g)Θ§ΗυΨίΗ«ΥΙΕ®¬…Θ§ΫΪΔΎΓΝ2-Δέ-ΔΌΓΝ2ΒΟΒΫΘ§2CH4(g)C2H4(g)+2H2(g)ΓςH=2ΓςH2-ΓςH3-2ΓςH1=+202.0kJ/molΘΜ
(2)”…Χβ“β““œ©”κ―θΤχ¥ΏΜ·÷Τ±ΗXΘ§XΒΡΖ÷Ή” ΫC2H4OΘ§≤ΜΚ§ΥΪΦϋΘ§Ζ¥”ΠΖϊΚœΉνάμœκΒΡ‘≠Ή”Ψ≠ΦΟΩ…÷ΣΘ§XΒΡΫαΙΙΦρ ΫΈΣ![]() ΘΜ
ΘΜ
(3)ΔΌ400Γφ ±Θ§œρ1LΒΡΚψ»ίΖ¥”ΠΤς÷–≥δ»κ1molCH4Θ§ΖΔ…ζ…œ ωΖ¥”ΠΘ§≤βΒΟΤΫΚβΜλΚœΤχΧε÷–C2H4ΒΡΧεΜΐΖ÷ ΐΈΣ25.0%Θ§…ηΉΣΜ·ΒΡΦΉΆιΈΣxΘ§”…¥ΥΫ®ΝΔ»γœ¬»ΐΕΈ ΫΘΚ
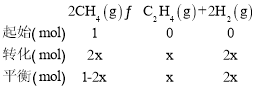
œύΆ§ΧθΦΰœ¬ΤχΧεΒΡΧεΜΐ±»Β»”ΎΈο÷ ΒΡΝΩ÷°±»Θ§Υυ“‘”–![]() =25%Θ§ΫβΒΟx=
=25%Θ§ΫβΒΟx=![]() molΘ§ΤΫΚβ ±ΤχΧεΒΡΉήΈο÷ ΒΡΝΩΈΣ
molΘ§ΤΫΚβ ±ΤχΧεΒΡΉήΈο÷ ΒΡΝΩΈΣ![]() Θ§»ίΤςΚψ―ΙΘ§‘ρΤχΧεΒΡΈο÷ ΒΡΝΩ÷°±»Β»”ΎΧεΜΐ÷°±»Θ§‘≠ΧεΜΐΈΣ1LΘ§‘ρ¥Υ ±ΧεΜΐ”ΠΈΣ
Θ§»ίΤςΚψ―ΙΘ§‘ρΤχΧεΒΡΈο÷ ΒΡΝΩ÷°±»Β»”ΎΧεΜΐ÷°±»Θ§‘≠ΧεΜΐΈΣ1LΘ§‘ρ¥Υ ±ΧεΜΐ”ΠΈΣ![]() LΘ§Υυ“‘Μ·―ßΤΫΚβ≥Θ ΐΈΣΤΫΚβ≥Θ ΐK=
LΘ§Υυ“‘Μ·―ßΤΫΚβ≥Θ ΐΈΣΤΫΚβ≥Θ ΐK=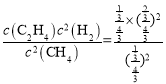 =1.0ΘΜ
=1.0ΘΜ
ΔΎΦΉΆι÷Τ±Η““œ©ΒΡΖ¥”ΠΈΣΤχΧεΧεΜΐ‘ω¥σΒΡΈϋ»»Ζ¥”ΠΘ§Ά®»κΗΏΈ¬Υ°’τΤχΘ§œύΒ±”ΎΦ”»»Θ§ΤΫΚβ”““ΤΘ§≤ζ¬ ‘ω¥σΘΜΆ§ ±Ά®»κΥ°’τΤχΘ§»ίΤςΒΡΧεΜΐ‘ω¥σΘ§œύΒ±”ΎΦθ–Γ―Ι«ΩΘ§ΤΫΚβ”““ΤΘ§≤ζ¬ “≤‘ω¥σΘ§“ρ¥ΥC2H4ΒΡ≤ζ¬ ΫΪ‘ω¥σΘΜ
Δέ»τ»ίΤςΧεΜΐΙΧΕ®Θ§ΦΉΆι÷Τ±Η““œ©ΒΡΖ¥”ΠΈΣΤχΧεΧεΜΐ‘ω¥σΒΡΖ¥”ΠΘ§Έ¬Ε»œύΆ§ ±Θ§‘ω¥σ―Ι«ΩΤΫΚβœρΡφΖ¥”ΠΖΫœρ“ΤΕ·Θ§CH4ΒΡΤΫΚβΉΣΜ·¬ ΫΒΒΆΘ§“ρ¥ΥP1>P2ΘΜ
(4) ΦΉΆι‘Ύ≤ΜΆ§Έ¬Ε»œ¬Ζ÷±π–Έ≥…![]() Β»Ή‘”…ΜυΘ§ΨίΆΦΩ…÷ΣΈ¬Ε»ΗΏ”Ύ600Γφ ±Θ§”Π”–ΫœΕύΒΡΉ‘”…Μυ
Β»Ή‘”…ΜυΘ§ΨίΆΦΩ…÷ΣΈ¬Ε»ΗΏ”Ύ600Γφ ±Θ§”Π”–ΫœΕύΒΡΉ‘”…Μυ![]() …ζ≥…Θ§Ή‘”…ΜυΫαΚœ…ζ≥…
…ζ≥…Θ§Ή‘”…ΜυΫαΚœ…ζ≥…![]() Θ§‘ρΥΪΧΦ”–ΜζΗ±≤ζΈοΈΣ““»≤ΘΜ
Θ§‘ρΥΪΧΦ”–ΜζΗ±≤ζΈοΈΣ““»≤ΘΜ
(5)»ΦΝœΒγ≥ΊΒΡΗΚΦΪΜ·ΚœΦέ…ΐΗΏΘ§C2H4‘ΎNaOH»ή“Κ÷–ΉΣΜ·ΈΣCO32-Θ§ΒγΦΪΖ¥”Π ΫΈΣΘΚC2H4-12e-+16OH-=2CO32-+10H2OΓΘ

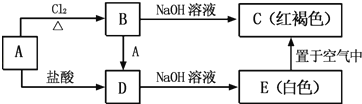
 ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ
ΩΈΧΟ»ΪΫβΉ÷¥ ΨδΕΈΤΣ’¬œΒΝ–¥πΑΗ ≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗ
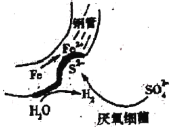
≤Ϋ≤ΫΗΏΩΎΥψΧβΩ®œΒΝ–¥πΑΗΓΨΧβΡΩΓΩΡ≥―–ΨΩ–ΓΉιΆ§―ß”ϊΧΫΨΩΡ≥¥ϋ≥®ΩΎΖ≈÷Ο“ΜΕΈ ±ΦδΒΡΟϊΈΣΓΑΝρΥα―«ΧζΦ“ΆΞ‘Α“’ΨΪΤΖΖ ΝœΓ±ΒΡΜ·Ζ ΒΡ÷ς“Σ≥…Ζ÷ΦΑœύΙΊ–‘÷ ΓΘ Ήœ»Ε‘ΗΟΜ·Ζ ΒΡ≥…Ζ÷Ϋχ––ΝΥ»γœ¬ΦΌ…ηΘΚ
aΘ°÷ΜΚ§”–FeSO4
bΘ°Κ§”–FeSO4ΚΆFe2(SO4)3
cΘ°÷ΜΚ§”–Fe2(SO4)3
ΫΪΜ·Ζ ΙΧΧεΖέΡ©»ή”ΎΥ°÷–ΒΟΒΫ»ή“Κ(Φ«ΈΣX)Θ§Ϋχ––»γœ¬ Β―ιΘΚ
Β―ι–ρΚ≈ | ≤ΌΉς | œ÷œσ |
ΔΓ | »Γ2 mL»ή“ΚXΘ§Φ”»κ1 mL 1 molΓΛLΘ≠1 NaOH»ή“Κ | ≤ζ…ζΚλΚ÷…Ϊ≥ΝΒμ |
ΔΔ | »Γ2 mL»ή“ΚXΘ§Φ”»κ1ΒΈKSCN | »ή“Κœ‘Κλ…Ϊ |
Θ®1Θ©«κ”ΟΈΡΉ÷±μ ωΉω≥ωΦΌ…ηbΒΡ“άΨί «__________________________ΓΘ
Θ®2Θ©Ε‘ Β―ιΔΓΒΡ‘ΛΤΎœ÷œσ «≤ζ…ζΑΉ…Ϊ≥ΝΒμΓΔ±δΈΣΜ“¬Χ…ΪΓΔΉνΚσ≥ωœ÷ΚλΚ÷…Ϊ≥ΝΒμΘ§‘ΛΤΎ≤ζ…ζΗΟœ÷œσΒΡ“άΨί «(”ΟΜ·―ßΖΫ≥Χ ΫΜράκΉ”ΖΫ≥Χ Ϋ±μ¥ο)_____ΓΔ_____ΓΘ
Θ®3Θ©”… Β―ιΔΔΒΟ≥ωΒΡΫα¬έ «____________ΓΘΫαΚœ Β―ιΔΓΓΔΔΔΘ§ΆΤ≤β Β―ιΔΓ ΒΦ œ÷œσ”κ‘ΛΤΎœ÷œσ≤ΜΖϊΒΡ‘≠“ρΩ…Ρή «_____________________________ΓΘΈΣΫχ“Μ≤Ϋ―ι÷ΛΦΌ…ηΘ§–ΓΉιΆ§―ßΫχ––ΝΥ“‘œ¬ Β―ιΘΚ
Β―ι–ρΚ≈ | ≤ΌΉς | œ÷œσ |
ΔΘ | »Γ2 mL»ή“ΚXΘ§Φ”»κ1ΒΈKSCNΘ§‘ΌΦ”»κ1 mLΥ° | »ή“Κœ‘Κλ…Ϊ |
ΔΛ | »Γ2 mL»ή“ΚXΘ§Φ”»κ1ΒΈKSCNΘ§‘ΌΦ”»κ1 mL¬»Υ° | »ή“Κœ‘Κλ…ΪΘ§―’…Ϊ±»ΔΘ…ν |
Θ®4Θ© Β―ιΔΛ÷–¬»Υ°≤ΈΦ”Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «_______________________ΓΘ
Θ®5Θ©Ά®Ιΐ“‘…œ Β―ιΘ§Ω…ΒΟΒΫΒΡΫα¬έ «_____________________________Θ§«κΆξ’ϊ±μ¥οΗΟΫα¬έ «»γΚΈΒΟ≥ωΒΡ_______________________________ΓΘ