��Ŀ����
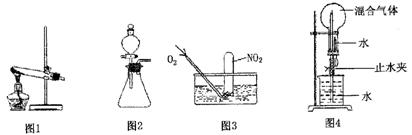
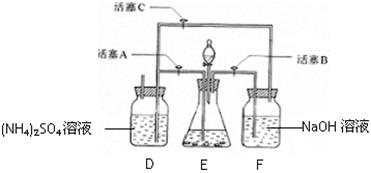
��.Ϊ��ȡ�ϴ������屽����֤��Ӧ������ij��ѧ����С�������ʵ��װ�ã�����ͼ����
�����Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��2��ʵ�����ʱ����A�¶˵Ļ������÷�Ӧ Һ����B�У������Ŀ����
Һ����B�У������Ŀ����  ��
��
��3��C��ʢ��CCl4�������� ��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤�����������һ�ַ���֤��֮��д�������������ۣ�
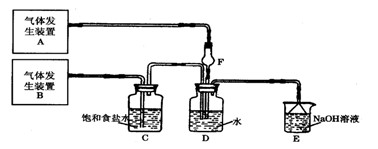
��.�����ӵĹ�ҵ��ˮ�����IJο��������£�
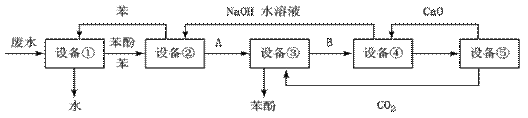
�ش��������⣺
��1���豸�ٽ��е��Dz���____________(��д��������)��ʵ������һ���������õ������ǣ�____________��
��2�����豸�ڽ����豸�۵�����A�� �����豸�۽����豸�ܵ�����B�� ��
��3�����豸���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
��4�����豸����, ����B��ˮ��Һ��CaO��Ӧ�������� �� ��ˮ����ͨ��________����(��д��������)������
��5����ͼ�У���ѭ��ʹ�õ�������C6H6��CaO��______��______��

�����Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��2��ʵ�����ʱ����A�¶˵Ļ������÷�Ӧ
 Һ����B�У������Ŀ����
Һ����B�У������Ŀ����  ��
����3��C��ʢ��CCl4�������� ��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤�����������һ�ַ���֤��֮��д�������������ۣ�
��.�����ӵĹ�ҵ��ˮ�����IJο��������£�

�ش��������⣺
��1���豸�ٽ��е��Dz���____________(��д��������)��ʵ������һ���������õ������ǣ�____________��
��2�����豸�ڽ����豸�۵�����A�� �����豸�۽����豸�ܵ�����B�� ��
��3�����豸���з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
��4�����豸����, ����B��ˮ��Һ��CaO��Ӧ�������� �� ��ˮ����ͨ��________����(��д��������)������
��5����ͼ�У���ѭ��ʹ�õ�������C6H6��CaO��______��______��
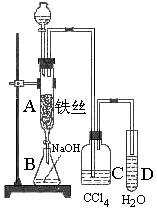
��1��
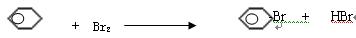
��2���ᴿ�屽
��3�� ��ȥHBr�е���
��4�����Թ�D�е��뼸����ɫʯ����Һ�������Һ�ʺ�ɫ��˵����HBr���ɣ������ķ�Ӧ��ȡ����Ӧ ��ÿ��2�ֹ�8�֣�
��.��3��С��2�֣�����ÿ��1�֣���11�֣���1����ȡ����Һ©�� ��2��C6H5ONa��NaHCO3
��3��C6H5ONa+CO2+H2O�� C6H5OH+NaHCO3 ��4��CaCO3��NaOH������
��5��NaOHˮ��Һ��CO2
��

��ϰ��ϵ�д�
�����Ŀ
 �� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ
�� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ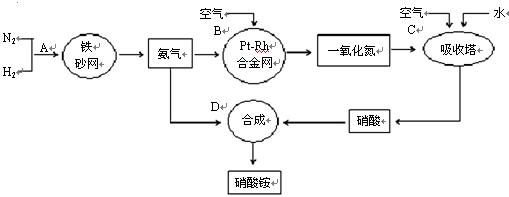
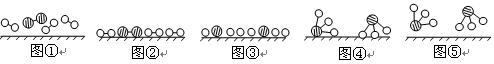
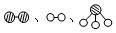 �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� ��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ��� �� �� 7N2+12H2O��NOҲ�����Ƶķ�Ӧ��
7N2+12H2O��NOҲ�����Ƶķ�Ӧ��