��Ŀ����
17��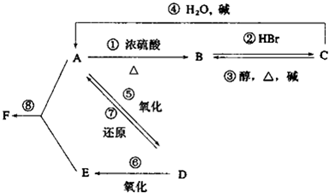 ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ��
ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ����֪D�������ܶ���������22���������Է���������Ӧ��
��1��A��F�Ľṹ��ʽ����ΪCH3CH2OH��CH2=CH2��CH3CH2Br��CH3CHO��CH3COOH��CH3COOC2H5��
��2���ڢ١����ת����������ȥ��Ӧ���Т٢ۼӳɷ�Ӧ���Тڢ�ȡ����Ӧ���Тܢ࣮
��3������A��F�У�ѡ���ʵ�����ĸ��գ���������������Cu��OH��2��Ӧ����E��Ŀǰ�ᳫ���ں����Ͱ�һ���������������������ȼ�ϵ���A��
��4���ֱ�д���٢ݵĻ�ѧ����ʽ��
��CH3CH2OH$��_{��}^{Ũ����}$CH2=CH2��+H2O��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� D�������ܶ���������22��������Է�������Ϊ44�������Է���������Ӧ��˵������-CHO����DΪCH3CHO����ת����ϵ��֪EΪCH3COOH��AΪCH3CH2OH��BΪCH2=CH2��CΪCH3CH2Br����FΪCH3COOC2H5������л���������Լ������ŵı仯�����⣮
��� �⣺D�������ܶ���������22��������Է�������Ϊ44�������Է���������Ӧ��˵������-CHO����DΪCH3CHO����ת����ϵ��֪EΪCH3COOH��AΪCH3CH2OH��BΪCH2=CH2��CΪCH3CH2Br����FΪCH3COOC2H5��
��1�������Ϸ�����֪AΪCH3CH2OH��BΪCH2=CH2��CΪCH3CH2Br��DΪCH3CHO��EΪCH3COOH��FΪCH3COOC2H5��
�ʴ�Ϊ��CH3CH2OH��CH2=CH2��CH3CH2Br��CH3CHO��CH3COOH��CH3COOC2H5��
��2��AΪCH3CH2OH��CΪCH3CH2Br�����߶�������B��BΪ����٢�Ϊ��ȥ��Ӧ��CH2=CH2��CH3CH2Br��CH3CHO��CH3CH2OH�ķ�ӦΪ�ӳɷ�Ӧ����ΪCH3CH2Br��CH3CH2OH����Ϊ�������Ҵ���������Ӧ��������ȡ����Ӧ��
�ʴ�Ϊ���٢ۣ��ڢߣ��ܢࣻ
��3����������������Cu��OH��2��Ӧ����CH3COOH��Ŀǰ�ᳫ���ں����Ͱ�һ���������������������ȼ�ϵ���CH3CH2OH��
�ʴ�Ϊ��E��A��
��4����Ӧ�ٵķ�ӦΪCH3CH2OH$��_{��}^{Ũ����}$CH2=CH2��+H2O����Ӧ�ݵķ���ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��CH3CH2OH$��_{��}^{Ũ����}$CH2=CH2��+H2O��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ���⿼���л�����ƶϣ���Ŀ�ѶȲ�����ע����DΪ�������ͻ�ƿڣ�������ʷ�Ӧ������ж����ʵ����࣮�����л�������ŵĽṹ�������ǽ�������Ŀ�Ĺؼ���

 ��һ���м�������˵����ȷ���ǣ�������
��һ���м�������˵����ȷ���ǣ�������| A�� | 1��3����ϩ����ʽΪC4H8 | |
| B�� | 1��3����ϩת��Ϊ ʱ������Cl2����1��4�ӳ���ˮ��õ� ʱ������Cl2����1��4�ӳ���ˮ��õ� | |
| C�� |  ��NaOH����Һ�л�Ũ�������ʱ���ȶ��ܷ�����ȥ��Ӧ ��NaOH����Һ�л�Ũ�������ʱ���ȶ��ܷ�����ȥ��Ӧ | |
| D�� |  ��������X��X�ܷ���������Ӧ����X�Ľṹֻ������ ��������X��X�ܷ���������Ӧ����X�Ľṹֻ������ |
| A�� | �� | B�� | ��ϩ | C�� | �Ҵ� | D�� | KMnO4��Һ |
| A�� | ��������X2ȫ��ת��Ϊ����ͬ��������X3����ôX3�ķ�������3�� | |
| B�� | ������Ϊ16��һ�ֺ����ڴ������е�ԭ�Ӱٷ���Ϊ80% | |
| C�� | ֻҪ������ͬλ����ɲ��䣬Xԭ�����κ������·���������ϣ����õ�����X2��������֮������15��4��1 | |
| D�� | ��������X2��ƽ����������32.6 |
| A�� | ���ӵ��ȶ����뻯ѧ����ǿ���� | |
| B�� | �μӷ�Ӧ�����������������������ڷ�Ӧ����ˮ�������� | |
| C�� | �Ͽ��μӷ�Ӧ�������������еĻ�ѧ�����յ�������������ˮʱ�γɻ�ѧ���ų������� | |
| D�� | �÷�Ӧ��Ҫ��ȼ�����������ȷ�Ӧ |
 ��
�� 
 +3HNO3 $��_{��}^{Ũ����}$
+3HNO3 $��_{��}^{Ũ����}$ +3H2O��
+3H2O��