��Ŀ����
2����ѧ����Դ����������������ʮ�ֹؼ������ã���1���̲��ں��ġ���ȼ�����Ǹ�ѹ���γɵ��������ļ���ˮ������壮��������ȼ�պ�ˮ�������Ȼ�ѧ����ʽ�ֱ�Ϊ��
CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802.3kJ•mol-1��
H2O��l���TH2O��g����H=+44kJ•mol-1��
��356g����ȼ����������ʽΪCH4•9H2O���ͷŵļ���������ȫȼ������Һ̬ˮ���ų�������Ϊ1780.6 kJ��
��2��0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ���������Ȼ�ѧ����ʽΪB2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165 kJ/mol��
��3������Һ��������Ҫ�ɷ�֮һ�Ƕ��飨C4H10������1g������ȫȼ�ղ�����CO2��Һ
̬ˮʱ���ų�����50kJ����д������ȼ���ȵ��Ȼ�ѧ����ʽC4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��l����H=-2900 kJ/mol��
���� ��1��356 g CH4•9H2O�����ͷų�2 mol CH4���ٸ���ȼ���ȵļ��㣻
��2��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ��������
��3��1g����������ȫȼ������CO2��H2O��l���ų�����50 kJ����58g���鷴Ӧ����CO2��H2O��l������2900kJ��
��� �⣺��1��356 g CH4•9H2O�����ͷų�2 mol CH4������CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802.3kJ•mol-1��
H2O��l���TH2O��g����H=+44kJ•mol-1����������ȼ����Ϊ890.3 kJ/mol����890.3 kJ/mol��2 mol=1 780.6 kJ��
�ʴ�Ϊ��1780.6��
��2��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ����������Ӧ���Ȼ�ѧ����ʽΪ��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165kJ/mol��
�ʴ�Ϊ��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165 kJ/mol��
��3��1g����������ȫȼ������CO2��H2O��l���ų�����50 kJ����58g���鷴Ӧ����CO2��H2O��l������2900kJ������ȼ�յ��Ȼ�ѧ����ʽΪ��C4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��l����H=-2900 kJ/mol��
�ʴ�Ϊ��C4H10��g��+$\frac{13}{2}$O2��g���T4CO2��g��+5H2O��l����H=-2900 kJ/mol��
���� ���⿼��ȼ���ȵ��Ȼ�ѧ����ʽ����д���ʱ����㣬��Ŀ�ѶȽϴ�ע���ʾ�Ȼ�ѧ����ʽ����дҪע�����ʾۼ�״̬�ͷ�Ӧ�ȵ����������⣮

| A�� | H��Be��Bԭ�������������������� | B�� | P��S��ClԪ����������ϼ����ν��� | ||
| C�� | C��N��O��Fԭ�Ӱ뾶�������� | D�� | Li��Na��K��Rb�Ľ��������μ��� |
| A�� | 9�� | B�� | 6 �� | C�� | 5�� | D�� | 8�� |
| A�� | 1 mol�ױ�����6NA��C-H�� | |
| B�� | 0.1 molCH2=CH-COOH�к���˫������ĿΪ0.1NA | |
| C�� | ��״���£�11.2 L���к��з��ӵ���ĿΪ0.5NA | |
| D�� | ��ϩ����ϩ��ɵ�56 g�����������ԭ�ӵĸ���Ϊ8NA |
 ��R��ʾ������R��R��ʾ�������⣩
��R��ʾ������R��R��ʾ�������⣩ ��
��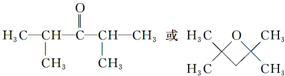 ����дһ�֣���
����дһ�֣���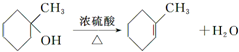 ��
��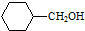 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2+CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2+CH3CH2Br$��_{��}^{NaOH��Һ}$CH3CH2OH��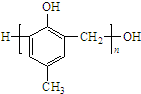 ���ĺϳ�·�����£�
���ĺϳ�·�����£�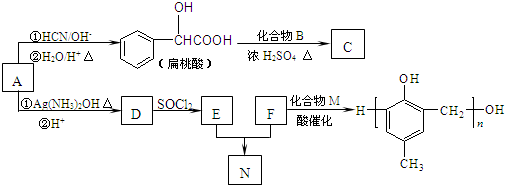
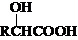
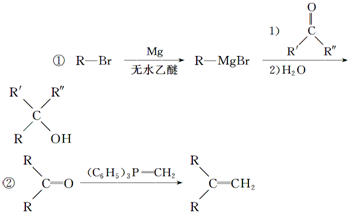
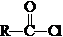 $\stackrel{R��OH}{��}$RCOOR�䣨R��R�����������
$\stackrel{R��OH}{��}$RCOOR�䣨R��R�����������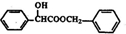 ��
��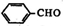 +2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$
+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$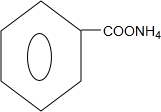 +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��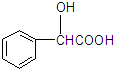 ���ж���ͬ���칹�壮���ڼ������Һ����ǻ���ͬ���칹�干��13�֣�д������һ�ֺ��Ǽ���-CH2-����ͬ���칹��Ľṹ��ʽ
���ж���ͬ���칹�壮���ڼ������Һ����ǻ���ͬ���칹�干��13�֣�д������һ�ֺ��Ǽ���-CH2-����ͬ���칹��Ľṹ��ʽ ��
��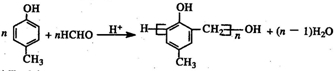 ��
��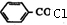 +
+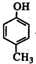 ��
��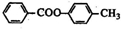 +HCl��
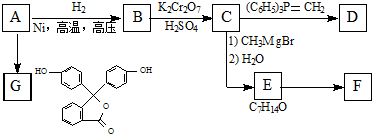
+HCl��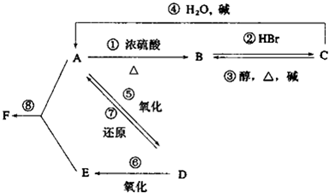 ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ��
ij�л���A����C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ��ͼ�� ��ͼ��ʾ�����һ��ԭ��أ��Իش��������⣨���ݹ��ʺ��ʣ���
��ͼ��ʾ�����һ��ԭ��أ��Իش��������⣨���ݹ��ʺ��ʣ���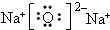 ��
��