��Ŀ����
���ơ�����������A1g��Ͷ��һ������ˮ�г�ַ�Ӧ������û��ʣ�࣬���ռ�����״���µ�����7.84L������Һ����μ���Ũ��Ϊ2mol?L-1��H2SO4��Һ����100mlʱ��ɫ�����ﵽ���������������H2SO4��Һ�����ټ���xmlH2SO4��Һʱ����ǡ����ʧ����������Һ�������յù���A2g�����н�����ȷ���м��֣�������
�ٳ����ﵽ�����ʱ��Һ�е�����ΪNa2SO4
��x=75
��A1=11.9��A2=17.1
�ܰ�ɫ���������Ϊ7.8g��
�ٳ����ﵽ�����ʱ��Һ�е�����ΪNa2SO4
��x=75
��A1=11.9��A2=17.1
�ܰ�ɫ���������Ϊ7.8g��
| A������ | B������ | C������ | D��һ�� |
���㣺��ѧ����ʽ���йؼ���,�йػ���ﷴӦ�ļ���
ר�⣺������
������������ΪA1g���ơ��������Ͷ��һ������ˮ�г�ַ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2Na+2H2O�T2NaOH+H2����2Al+2NaOH+2H2O�T2NaAlO2+3H2��������û��ʣ�࣬���ռ�����״���µ�����7.84L��
��Ӧ�����Һ�м�������100mlʱ��ɫ�����ﵽ���������ʱ��Һ������ΪNa2SO4�����ݴ�ʱ���ĵ��������n��Na�����ٸ������ɵ����壬���õ���ת���غ����n��Al��������m=nM�����Ͻ��������������ٸ���Al�غ���Լ������������������������
��������H2SO4��Һ�����ټ���xmlH2SO4��Һʱ����ǡ����ʧ����ʱ��Һ�ɷ���Na2SO4��Al2��SO4��3��������ԭ�ӡ���ԭ�ӡ�������غ�������ǡ����ʧʱ���ĵ����ᣬ��������x��ֵ��
������������Һ�������յù���ΪNa2SO4��Al2��SO4��3��������=m��Na��+m��Al��+m��SO42-�����ݴ˽��
��Ӧ�����Һ�м�������100mlʱ��ɫ�����ﵽ���������ʱ��Һ������ΪNa2SO4�����ݴ�ʱ���ĵ��������n��Na�����ٸ������ɵ����壬���õ���ת���غ����n��Al��������m=nM�����Ͻ��������������ٸ���Al�غ���Լ������������������������
��������H2SO4��Һ�����ټ���xmlH2SO4��Һʱ����ǡ����ʧ����ʱ��Һ�ɷ���Na2SO4��Al2��SO4��3��������ԭ�ӡ���ԭ�ӡ�������غ�������ǡ����ʧʱ���ĵ����ᣬ��������x��ֵ��
������������Һ�������յù���ΪNa2SO4��Al2��SO4��3��������=m��Na��+m��Al��+m��SO42-�����ݴ˽��
���
�⣺������ΪA1g���ơ��������Ͷ��һ������ˮ�г�ַ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2Na+2H2O�T2NaOH+H2����2Al+2NaOH+2H2O�T2NaAlO2+3H2����
��Ӧ�����Һ�м�������100mlʱ��ɫ�����ﵽ���������ʱ��Һ������ΪNa2SO4����ʱ���ĵ�����Ϊ0.1L��2mol/L=0.2mol����n��Na��=2n��Na2SO4��=2n��H2SO4��=0.4mol��
����û��ʣ�࣬���ռ�����״���µ�����7.84L�������ʵ���=
=0.35mol�����ݵ���ת���غ㣺n��Al��=
=0.1mol���ʽ�����������A1=23g/mol��0.4mol+27g/mol��0.1mol=11.9g��
����ԭ���غ�֪�����������ʱ��n[Al��OH��3]=n��Al��0.1mol����m[Al��OH��3]=0.1mol��78g/mol=7.8g��
��������H2SO4��Һ�����ټ���xmlH2SO4��Һʱ����ǡ����ʧ����ʱ��Һ�ɷ���Na2SO4��Al2��SO4��3��������ԭ�ӡ���ԭ�ӡ�������غ㣬��֪����ǡ����ʧʱ���ĵ�����Ϊ��n��H2SO4��=n��Na2SO4��+3n[Al2��SO4��3]=0.2mol+3����0.1mol��
��=0.35mol���ʴ�ʱ�������������=
=0.175L=175mL����x=175mL-100mL=75mL��
������������Һ�������յù���ΪNa2SO4��Al2��SO4��3��������=m��Na��+m��Al��+m��SO42-��=11.9g+0.35mol��96g/mol=45.5g��
���Ϸ�����֪���٢ڢ���ȷ���۴���ѡB��
��Ӧ�����Һ�м�������100mlʱ��ɫ�����ﵽ���������ʱ��Һ������ΪNa2SO4����ʱ���ĵ�����Ϊ0.1L��2mol/L=0.2mol����n��Na��=2n��Na2SO4��=2n��H2SO4��=0.4mol��
����û��ʣ�࣬���ռ�����״���µ�����7.84L�������ʵ���=
| 7.84L |
| 22.4L/mol |
| 0.35mol��2-0.4mol��1 |
| 3 |
����ԭ���غ�֪�����������ʱ��n[Al��OH��3]=n��Al��0.1mol����m[Al��OH��3]=0.1mol��78g/mol=7.8g��
��������H2SO4��Һ�����ټ���xmlH2SO4��Һʱ����ǡ����ʧ����ʱ��Һ�ɷ���Na2SO4��Al2��SO4��3��������ԭ�ӡ���ԭ�ӡ�������غ㣬��֪����ǡ����ʧʱ���ĵ�����Ϊ��n��H2SO4��=n��Na2SO4��+3n[Al2��SO4��3]=0.2mol+3����0.1mol��
| 1 |
| 2 |
| 0.35mol |
| 2mol/L |
������������Һ�������յù���ΪNa2SO4��Al2��SO4��3��������=m��Na��+m��Al��+m��SO42-��=11.9g+0.35mol��96g/mol=45.5g��
���Ϸ�����֪���٢ڢ���ȷ���۴���ѡB��
���������⿼���˻������йؼ��㣬��ȷ��Ӧ�����з����ķ�Ӧ�ǽⱾ��ؼ���ע�������غ�˼����н��ע����������Һ���ɡ�����ʱ�õ������������������Ȼ�����Һ��������ʱ�õ���������������Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
ʵ������Cl2��ӦΪMnO2+4HCl��Ũ��
MnCl2+Cl2��+2H2O������˵������ȷ���ǣ�������
| ||
| A����������MnO2����ԭ����HCl |
| B��ÿ����1mol Cl2��ת�Ƶ��ӵ����ʵ���Ϊ2mol |
| C��ÿ����1mol MnO2����ԭ�����õ�HCl����4mol |
| D��ת�Ƶ��ӵ����ʵ���Ϊ1molʱ�����ɱ�״����Cl2�����Ϊ11.2L |
�����£�ͨ���ⶨ������ܶȿ���������������Ħ���������������ϴ���ǣ�������
| A��CO2 |
| B��H2 |
| C��NO2 |
| D��CH4 |
�����й����ʵ����ʺ����ʵ�Ӧ�þ���ȷ���ǣ�������
| A��������Ũ����������������Ӧ�����ڳ�����������������������Ũ���� |
| B���������費���κ��ᷴӦ������ʯӢ������������ |
| C���������Ⱦ��������ԣ�����������ˮ��ɱ������ |
| D��ͭ�Ľ��������Ա������������ں��������װ����ͭ���Լ����丯ʴ |
�����ҹ��ж���������������������������PM2.5�����ݼ�����빫����Ұ��PM2.5��ָ������ֱ��С�ڻ����2.5�ף�1��=10-6�ף��Ŀ���ε��к������������й�˵������ȷ���ǣ�������
| A��PM2.5��������������������������� |
| B��PM2.5�ڿ�����һ�����γ����ܽ� |
| C����ϴֵĴ�����������ȣ�PM2.5�����彡���ʹ�������������Ӱ����� |
| D���ո�ȼ�ա�ú̿ȼ�ա�����β�����ŷŵȶ���PM2.5���γ��й� |
��ͼ��ʾװ�ã������Ƶ�ָ�뷢��ƫת��������֣�������ϸ����������������ǣ�������
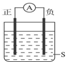

| A������Cu������Zn��SΪϡH2SO4 |
| B������Zn������Cu��SΪCuSO4��Һ |
| C������Ag������Zn��SΪAgNO3��Һ |
| D������Fe������Cu��SΪAgNO3��Һ |
�����з������Ʊ��ܽ�����0.5mol/L BaCl2��Һ�͵����2mol/L�������ϲ����ڰ�5��6�α������Ȼ�����Һ�μ���20mL��ˮ�У��ӱ����۽�������μ��뵽����Na2SiO3��Һ�У����������е��ǣ�������
| A���٢� | B���٢� | C���ڢ� | D���٢ڢ� |
���з�Ӧ�����ӷ���ʽ��ʾ��ȷ���ǣ�������
| A����Fe��NO3��3��Һ�м��������HI��Һ��2NO3-+8H++6I-�T3I2+2NO��+4H2O |
| B��������Һ�м�����������������Һ��Ba2++OH-+H++SO42-�TBaSO4��+H2O |
| C���������ȣ�SOCl2������ˮ�����������SOCl2+2H2O�TH2SO3+2H++2Cl- |
| D����̼��������Ũ���Ṳ�ȷ�Ӧ����������ͨ���������������Һ�У�CO2+SO2+4OH-�TSO32-+CO32-+2H2O |
һ�������£�X��g��+3Y��g��?2Z��g���ﵽƽ��ı�־�ǣ�������
| A��Z�ķֽ����ʺ������������ |
| B��X��Y��Z��Ũ�ȱ�Ϊ1��3��2 |
| C����Ӧ��ϵ�����������ֲ��� |
| D����λʱ��������n mol Z��ͬʱ����n mol X |