��Ŀ����
��14�֣�����̼���ƺ��Ȼ��ƵĹ������Ϊ�˲ⶨ��Ʒ��̼���Ƶĺ�������ѧ��ȤС��ͬѧ���������������ʵ�顣
��һ��
ʵ��װ��
ʵ�鲽��
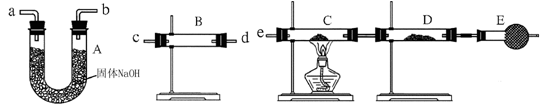
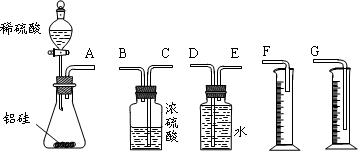
�����Ӻ�װ�ã���������ԣ�
��ʢװҩƷ��������Ʒa g������E�����������Ӻ�װ�ã�
�۹ر�ֹˮ�У���B�м���һ����ϡ���
�ܵ�B�г�ַ�Ӧ��ֹˮ�У�����Aװ�ã�ͨ��һ�����Ŀ�������B��D�в��������ȫ�����뵽Eװ���У�
�ݳ���E��������b g ��
�ش��������⣺
��1������C������ ��D��ʢ�ŵ��Լ��� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��A�з�Ӧ�����ӷ���ʽΪ ��
��4���ı�����һ�����������²ⶨ��Ʒ�е�̼���Ƶ������ٷֺ���ƫ�͵��� ��ѡ����ĸ��
A.ʵ����ڲ�ͨ����� B.������C�е����ỻ������
C.��������D D.��������F
�ڶ���
ʵ��װ��
ʵ�鲽��
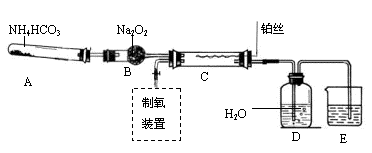
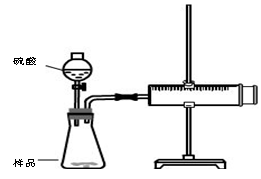
�ټ��װ��������
��װ���Լ���������Ʒb g,���Ӻ�װ�ã���ע�����Ļ����Ƶ��ף���0mL��
�ۼ���20mL���ᣬ
�ܴ���Ӧ��ֽ��к��������ƶ������»���ǰ�˶�Ӧ�Ŀ̶�ΪV mL
�ش���������
����װ�������Եķ����ǣ� ��
��2�����ڲ����������������ֹ۵㣬�۵�һ��������������ΪVmL���۵����������������Ϊ(V-20)mL������Ϊ ����۵�һ�����۵��������ȷ��
��3�������ʵ���ڱ�״���½��У�b=0.5g��V=76mL������Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
��һ��
ʵ��װ��

ʵ�鲽��
�����Ӻ�װ�ã���������ԣ�
��ʢװҩƷ��������Ʒa g������E�����������Ӻ�װ�ã�
�۹ر�ֹˮ�У���B�м���һ����ϡ���
�ܵ�B�г�ַ�Ӧ��ֹˮ�У�����Aװ�ã�ͨ��һ�����Ŀ�������B��D�в��������ȫ�����뵽Eװ���У�
�ݳ���E��������b g ��
�ش��������⣺
��1������C������ ��D��ʢ�ŵ��Լ��� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3��A�з�Ӧ�����ӷ���ʽΪ ��
��4���ı�����һ�����������²ⶨ��Ʒ�е�̼���Ƶ������ٷֺ���ƫ�͵��� ��ѡ����ĸ��
A.ʵ����ڲ�ͨ����� B.������C�е����ỻ������
C.��������D D.��������F
�ڶ���
ʵ��װ��

ʵ�鲽��
�ټ��װ��������
��װ���Լ���������Ʒb g,���Ӻ�װ�ã���ע�����Ļ����Ƶ��ף���0mL��
�ۼ���20mL���ᣬ
�ܴ���Ӧ��ֽ��к��������ƶ������»���ǰ�˶�Ӧ�Ŀ̶�ΪV mL
�ش���������
����װ�������Եķ����ǣ� ��
��2�����ڲ����������������ֹ۵㣬�۵�һ��������������ΪVmL���۵����������������Ϊ(V-20)mL������Ϊ ����۵�һ�����۵��������ȷ��
��3�������ʵ���ڱ�״���½��У�b=0.5g��V=76mL������Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
��һ��
��1����Һ©����1�֣���Ũ���ᣨ1�֣� ��2��Na2CO3+H2SO4=Na2SO4+CO2��+H2O (2��)
��3��CO2+2OH��=CO32��+H2O (2��) ��4��A (2��)
�ڶ���
��1�����Ӻ�װ�ã��رշ�Һ©���Ļ�������ע�����Ļ���������һ�ξ��룬�ɿ����������������ջص�ԭ����λ�ã���֤�����������ã����������Բ��á���2�֣�
��2���۵����2�֣� (3)53%��2�֣�
��1����Һ©����1�֣���Ũ���ᣨ1�֣� ��2��Na2CO3+H2SO4=Na2SO4+CO2��+H2O (2��)
��3��CO2+2OH��=CO32��+H2O (2��) ��4��A (2��)
�ڶ���
��1�����Ӻ�װ�ã��رշ�Һ©���Ļ�������ע�����Ļ���������һ�ξ��룬�ɿ����������������ջص�ԭ����λ�ã���֤�����������ã����������Բ��á���2�֣�
��2���۵����2�֣� (3)53%��2�֣�
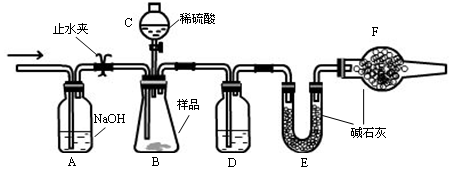
��һ��ʵ�飺����CΪ��Һ©�������Ĺ�����������ͨ©������ͨ©��û�л�����Ҳû��ƿ��������Һ©���С���ϡ���������Ʒ��ϡ�����������Ʒ�е�̼���Ʒ������ֽⷴӦ���仯ѧ��Ӧ����ʽΪ��H2SO4 + Na2CO3===== Na2SO4 + CO2��+ H2O����װ��B�г�����CO2�����к���ˮ������Ϊ�˷�ֹˮ������Eװ���еļ�ʯ�����ն������Ʒ��Na2CO3�������ٷֺ���ƫ�ߣ�Dװ��Ҫ��ʢ��ŨH2SO4��ϴ��ƿ��ȥCO2�����к���ˮ������Ϊ�˷�ֹ�����е�CO2�����ˮ��������Eװ�ö������Ʒ��Na2CO3�������ٷֺ���ƫ�ߣ����ԣ�����Ҫ��װ�õ��������һ��ʢ�м�ʯ�ҵ����θ���ܡ�
��Ӧ������Ϊ��ʹ���ɵ�CO2�����ֱ�Eװ���еļ�ʯ�����գ���Ҫ��Aװ���л���ͨ�������ʹװ���в�����CO2����ȫ����Eװ���еļ�ʯ�����ա����ڿ�����Ҳ����һ����CO2���壬Ϊ�˷�ֹ�����е�CO2��Eװ���еļ�ʯ�����գ����ԣ���Aװ���м�������������Һ����ȥ�����е�CO2���壬���ӷ���ʽΪ��CO2+2OH��=CO32��+H2O����Ȼ��ϡ�������������棬��Ϊ�����ӷ���HCL���壬Ҳ�ᱻ��ʯ�����գ��Ӷ����Na2CO3�������ٷ���ƫ�ߡ�������������һ��ʵ�����ȷ��Ϊ��
��һ��
��1����Һ©����1�֣���Ũ���ᣨ1�֣� ��2��Na2CO3+H2SO4=Na2SO4+CO2��+H2O (2��)
��3��CO2+2OH��=CO32��+H2O (2��) ��4��A (2��)
�ڶ���ʵ���������1�����Ӻ�װ�ã��رշ�Һ©���Ļ�������ע�����Ļ���������һ�ξ��룬�ɿ����������������ջص�ԭ����λ�ã���֤�����������ã����������Բ��á�
��2��������20mL�������Ҳ�ᱻ����ע�����У����Թ۵����ȷ��
��3����״���£�������������76mL�������������ʵ����������������Ʒ��̼���Ƶ������ٷֺ���Ϊ53%
��Ӧ������Ϊ��ʹ���ɵ�CO2�����ֱ�Eװ���еļ�ʯ�����գ���Ҫ��Aװ���л���ͨ�������ʹװ���в�����CO2����ȫ����Eװ���еļ�ʯ�����ա����ڿ�����Ҳ����һ����CO2���壬Ϊ�˷�ֹ�����е�CO2��Eװ���еļ�ʯ�����գ����ԣ���Aװ���м�������������Һ����ȥ�����е�CO2���壬���ӷ���ʽΪ��CO2+2OH��=CO32��+H2O����Ȼ��ϡ�������������棬��Ϊ�����ӷ���HCL���壬Ҳ�ᱻ��ʯ�����գ��Ӷ����Na2CO3�������ٷ���ƫ�ߡ�������������һ��ʵ�����ȷ��Ϊ��
��һ��
��1����Һ©����1�֣���Ũ���ᣨ1�֣� ��2��Na2CO3+H2SO4=Na2SO4+CO2��+H2O (2��)
��3��CO2+2OH��=CO32��+H2O (2��) ��4��A (2��)
�ڶ���ʵ���������1�����Ӻ�װ�ã��رշ�Һ©���Ļ�������ע�����Ļ���������һ�ξ��룬�ɿ����������������ջص�ԭ����λ�ã���֤�����������ã����������Բ��á�
��2��������20mL�������Ҳ�ᱻ����ע�����У����Թ۵����ȷ��
��3����״���£�������������76mL�������������ʵ����������������Ʒ��̼���Ƶ������ٷֺ���Ϊ53%

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ