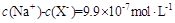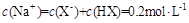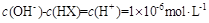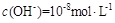��Ŀ����
�����ƶϻ������ȷ���� �� ��
2c��Na+�� 3[c��HCO3����+c��CO32����+c��H2CO3��]
| A���ֱ������Ϊ1L��1mol/L NaCI��Һ��1mol/L��NaF��Һ��ˮϡ����100L������Һ����������� |
| B����pH =a�Ĵ�����Һϡ��100����ϡ��ҺpH=b����b=a+2 |
| C��0.2mol/L��������0.05mol/L��Ba�� OH��2��Һ�������ϣ����ҺpH =1 |
| D����1L��0.3mol/L��NaOH��Һ����ͨ��CO2���壬����Һ��������8.8g������Һ�У� |
D
���������A��NaF��ǿ�������Σ��ᷢ��ˮ�⣬�ʴ���B�����������ᣬ������ʣ����ڵ���ƽ�⣬b<a+2���ʴ���C�������Һ��Ũ��Ϊ0.05mol/L���ʴ�����ѡD��
���������⿼����������ˮ�⡢����ƽ�⡢pH�����֪ʶ����Ŀ�Ѷ��У���Ϥ����ƽ���ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
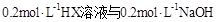 ��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH��8��������˵�������ϵʽ����ȷ����
��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH��8��������˵�������ϵʽ����ȷ����