ΧβΡΩΡΎ»ί
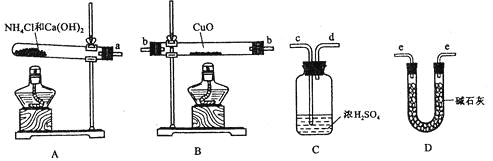
ΓΨΧβΡΩΓΩœρ“ΜΧεΜΐ≤Μ±δΒΡΟή±’»ίΤς÷–Φ”»κ2 mol AΓΔ0.6 mol CΚΆ“ΜΕ®ΝΩΒΡB»ΐ÷÷ΤχΧεΓΘ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”ΠΘ§ΗςΈο÷ ≈®Ε»Υφ ±Φδ±δΜ·»γΆΦ“ΜΥυ ΨΓΘΆΦΕΰΈΣt2 ±ΩΧΚσΗΡ±δΖ¥”ΠΧθΦΰΘ§ΤΫΚβΧεœΒ÷–Ζ¥”ΠΥΌ¬ Υφ ±Φδ±δΜ·ΒΡ«ιΩωΘ§«“ΥΡΗωΫΉΕΈΕΦΗςΗΡ±δ“Μ÷÷≤ΜΆ§ΒΡΧθΦΰΓΘ“―÷Σt3Θ≠t4ΫΉΕΈΈΣ Ι”Ο¥ΏΜ·ΦΝΘΜΆΦ“Μ÷–t0Θ≠t1ΫΉΕΈc(B)Έ¥Μ≠≥ωΓΘ
œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «
A.¥ΥΈ¬Ε»œ¬ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ2A(g)ΘΪB(s)![]() 3C(g)
3C(g)
B.t4Θ≠t5ΫΉΕΈΗΡ±δΒΡΧθΦΰΈΣΦθ–Γ―Ι«Ω
C.BΒΡΤπ ΦΈο÷ ΒΡΝΩΈΣ1.0 mol
D.‘ΎœύΆ§ΧθΦΰœ¬Θ§»τΤπ Φ ±»ίΤς÷–Φ”»κa mol AΓΔb mol BΚΆc mol CΘ§“Σ¥οΒΫt1 ±ΩΧΆ§―υΒΡΤΫΚβΘ§aΓΔbΓΔc“Σ¬ζΉψΒΡΧθΦΰΈΣaΘΪ2c/3=2.4ΚΆbΘΪc/3=1.2
ΓΨ¥πΑΗΓΩA
ΓΨΫβΈωΓΩ
AΘ°t3ΓΪt4ΫΉΕΈ”κt4ΓΪt5ΫΉΕΈ’ΐΡφΖ¥”ΠΥΌ¬ ΕΦœύΒ»Θ§Εχt3ΓΪt4ΫΉΕΈΈΣ Ι”Ο¥ΏΜ·ΦΝΘ§‘ρt4ΓΪt5ΫΉΕΈ”ΠΈΣΦθ–Γ―Ι«ΩΘ§Φ¥ΗΟΖ¥”ΠΈΣΤχΧεΧεΜΐ≤Μ±δΒΡΖ¥”ΠΘΜΖ¥”Π÷–AΒΡ≈®Ε»±δΜ·ΈΣ1mol/L-0.8mol/L=0.2mol/LΘ§CΒΡ≈®Ε»±δΜ·ΈΣ0.6mol/L-0.3mol/L=0.3mol/LΘ§‘ρAΓΔCΒΡΜ·―ßΦΤΝΩ ΐ÷°±»ΈΣ2ΘΚ3Θ§”÷ΗΟΖ¥”ΠΈΣΤχΧεΧεΜΐ≤Μ±δΒΡΖ¥”ΠΘ§‘ρBΈΣΖ¥”ΠΈοΘ§ΗΟΖ¥”ΠΈΣ2A(g)+B(g)![]() 3C(g)Θ§Ι A¥μΈσΘΜ
3C(g)Θ§Ι A¥μΈσΘΜ
BΘ°t4ΓΪt5ΫΉΕΈΗΡ±δΒΡΧθΦΰΈΣΦθ–Γ―Ι«ΩΘ§ΗΟΖ¥”ΠΈΣΤχΧεΧεΜΐ≤Μ±δΒΡΖ¥”ΠΘ§Ι B’ΐ»ΖΘΜ
CΘ°AΦθ…Ό0.2mol/LΘ§‘ρBΦθ…Ό0.1mol/LΘ§‘ρΤπ ΦBΒΡ≈®Ε»ΈΣ0.1mol/L+0.4mol/L=0.5mol/LΘ§Τπ ΦΈο÷ ΒΡΝΩΈΣ0.5mol/LΓΝ2L=1molΘ§Ι C’ΐ»ΖΘΜ
DΘ°œύΆ§ΧθΦΰœ¬Θ§¥οΒΫΆ§―υΒΡΤΫΚβΉ¥Χ§Θ§‘ρΤπ ΦΝΩœύΆ§Φ¥Ω…Θ§‘ρΉΣΜ·Κσ¬ζΉψΒΡΧθΦΰΈΣa+![]() =2.4ΚΆb+
=2.4ΚΆb+![]() =1.2Θ§Ι D’ΐ»ΖΘΜ
=1.2Θ§Ι D’ΐ»ΖΘΜ
Ι ¥πΑΗΈΣAΓΘ

ΓΨΧβΡΩΓΩΡ≥ Β―ι–ΓΉι”Ο0.50molΓΛLΘ≠1 NaOH»ή“ΚΚΆ0.50molΓΛLΘ≠1ΝρΥα»ή“ΚΫχ––Ζ¥”Π»»ΒΡ≤βΕ®ΓΘ
Θ®1Θ©–¥≥ωΗΟΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ[…ζ≥…1 molH2O(l) ±ΒΡΖ¥”Π»»ΈΣΘ≠57.3 kJΓΛmolΘ≠1]ΘΚ___ΓΘ
Θ®2Θ©»Γ50 mLNaOH»ή“ΚΚΆ30 mL ΝρΥα»ή“ΚΫχ–– Β―ιΘ§ Β―ι ΐΨί»γ±μΓΘ
ΔΌ«κΧν–¥±μ÷–ΒΡΩ’ΑΉΘΚ
Έ¬Ε» ¥Έ ΐΓΓΓΓ | Τπ ΦΈ¬Ε»t1/Γφ | ÷’÷ΙΈ¬Ε»t2/Γφ | Έ¬Ε»≤νΤΫΨυ÷Β(t2Θ≠t1)/Γφ | ||
H2SO4 | NaOH | ΤΫΨυ÷Β | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ___ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
ΔΎΫϋΥΤ»œΈΣ0.50molΓΛLΘ≠1NaOH»ή“ΚΚΆ0.50molΓΛLΘ≠1ΝρΥα»ή“ΚΒΡΟήΕ»ΕΦ «1.0gΓΛmLΘ≠1Θ§÷–ΚΆΚσ…ζ≥…»ή“ΚΒΡ±»»»»ίcΘΫ4.18J/(gΓΛΓφ)ΓΘ‘ρ…ζ≥…1 mol H2O(l) ±ΒΡΖ¥”Π»»ΠΛHΘΫ___(»Γ–Γ ΐΒψΚσ“ΜΈΜ)ΓΘ
Δέ…œ ω Β―ι ΐ÷ΒΫαΙϊ”κΘ≠57.3kJΓΛmolΘ≠1”–ΤΪ≤νΘ§≤ζ…ζΤΪ≤νΒΡ‘≠“ρ≤ΜΩ…Ρή «(ΧνΉ÷ΡΗ)___ΓΘ
aΘ° Β―ιΉΑ÷Ο±ΘΈ¬ΓΔΗτ»»–ßΙϊ≤ν
bΘ°ΝΩ»ΓNaOH»ή“ΚΒΡΧεΜΐ ±―ω ”ΕΝ ΐ
cΘ°Ζ÷Εύ¥ΈΑ―NaOH»ή“ΚΒΙ»κ Δ”–ΝρΥαΒΡ–Γ…’±≠÷–
dΘ°”ΟΈ¬Ε»ΦΤ≤βΕ®NaOH»ή“ΚΤπ ΦΈ¬Ε»Κσ÷±Ϋ”≤βΕ®H2SO4»ή“ΚΒΡΈ¬Ε»
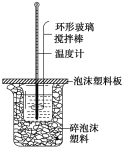
ΓΨΧβΡΩΓΩΡ≥…’Φν―υΤΖΚ§”–…ΌΝΩ≤Μ”κΥαΉς”ΟΒΡ‘”÷ ,ΈΣΝΥ≤βΕ®Τδ¥ΩΕ»,Ϋχ––“‘œ¬ΒΈΕ®≤ΌΉςΘΚ
A. ‘Ύ250 mLΒΡ»ίΝΩΤΩ÷–Ε®»ί≈δ÷Τ250 mL…’Φν»ή“ΚΘΜ
B. ”ΟΦν ΫΒΈΕ®Ιή“Τ»Γ25.00 mL…’Φν»ή“Κ”ΎΉΕ–ΈΤΩ÷–≤ΔΒΈ»κ2ΒΈΦΉΜυ≥»÷Η ΨΦΝΘΜ
C. ‘ΎΧλΤΫ…œΉΦ»Ζ≥Τ»Γ…’Φν―υΤΖ2.0 g,‘Ύ…’±≠÷–”Ο’τΝσΥ°»ήΫβΘΜ
D. ΫΪΈο÷ ΒΡΝΩ≈®Ε»ΈΣ0.100 0 molΓΛLΘ≠1ΒΡ±ξΉΦ―ΈΥαΉΑ»κΥα ΫΒΈΕ®Ιή,Βς’ϊ“ΚΟφΦ«œ¬ΩΣ ΦΕΝ ΐΈΣV1ΘΜ
E. ‘ΎΉΕ–ΈΤΩœ¬Βφ“Μ’≈ΑΉ÷Ϋ,ΒΈΕ®÷Ν÷’Βψ,Φ«œ¬ΕΝ ΐV2ΓΘ
ΨΆ¥Υ Β―ιΆξ≥…œ¬Ν–ΧνΩ’ΘΚ
Θ®1Θ©’ΐ»ΖΒΡ≤ΌΉς≤Ϋ÷ηΒΡΥ≥–ρ «(”Ο±ύΚ≈Ή÷ΡΗΧν–¥)
________Γζ________Γζ________ΓζDΓζ________ΓΘ
Θ®2Θ©…œ ωE÷–ΉΕ–ΈΤΩœ¬Βφ“Μ’≈ΑΉ÷ΫΒΡΉς”Ο «_______________________________
Θ®3Θ©”Ο±ξΉΦΒΡ―ΈΥαΒΈΕ®¥ΐ≤βΒΡNaOH»ή“Κ ±Θ§Ήσ ÷Έ’Υα ΫΒΈΕ®ΙήΒΡΜν»ϊΘ§”“ ÷“ΓΕ·ΉΕ–ΈΤΩΘ§―έΨΠΉΔ ”______________ΓΘ÷±ΒΫΦ”»κ“ΜΒΈ―ΈΥαΚσΘ§»ή“Κ____________________________ (Χν―’…Ϊ±δΜ·)ΓΘ
Θ®4Θ©œ¬Ν–≤ΌΉς÷–Ω…Ρή ΙΥυ≤βNaOH»ή“ΚΒΡ≈®Ε» ΐ÷ΒΤΪΒΆΒΡ «Θ®____________Θ©
A.Υα ΫΒΈΕ®ΙήΈ¥”Ο±ξΉΦ―ΈΥα»σœ¥ΨΆ÷±Ϋ”ΉΔ»κ±ξΉΦ―ΈΥα
B.ΒΈΕ®«Α ΔΖ≈NaOH»ή“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσΟΜ”–Η…‘ο
C.Υα ΫΒΈΕ®Ιή‘ΎΒΈΕ®«Α”–Τχ≈ίΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ß
D.ΕΝ»Γ―ΈΥαΧεΜΐ ±Θ§ΩΣ Φ―ω ”ΕΝ ΐΘ§ΒΈΕ®Ϋα χ ±Η© ”ΕΝ ΐ
Θ®5Θ©»τΒΈΕ®ΩΣ ΦΚΆΫα χ ±Θ§Υα ΫΒΈΕ®Ιή÷–ΒΡ“ΚΟφ»γΆΦΥυ ΨΘ§‘ρΥυ”Ο―ΈΥα»ή“ΚΒΡΧεΜΐΈΣ________mLΓΘ
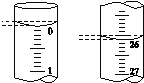
Θ®6Θ©Ρ≥―ß…ζΗυΨί3¥Έ Β―ιΖ÷±πΦ«¬Φ”–ΙΊ ΐΨί»γ±μΘΚ
ΒΈΕ® ¥Έ ΐ | ¥ΐ≤βNaOH»ή“ΚΒΡΧεΜΐ/mL | 0.100 0 molΓΛLΘ≠1―ΈΥαΒΡΧεΜΐ/mL | ||
ΒΈΕ®«Α ΩΧΕ» | ΒΈΕ®ΚσΩΧΕ» | »ή“ΚΧεΜΐ/mL | ||
ΒΎ“Μ¥Έ | 25.00 | 0.00 | 26.11 | 26.11 |
ΒΎΕΰ¥Έ | 25.00 | 1.56 | 30.30 | 28.74 |
ΒΎ»ΐ¥Έ | 25.00 | 0.22 | 26.31 | 26.09 |
“άΨί…œ±μ ΐΨίΝ– ΫΦΤΥψΗΟ…’ΦνΒΡ¥ΩΕ»____ΓΘΘ®ΫαΙϊ±ΘΝτΥΡΈΜ”––ß ΐΉ÷Θ©