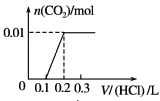
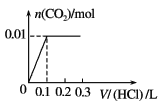
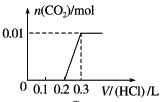
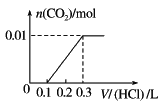
��Ŀ����
����Ŀ����1����֪C(s�����ʯ��+O2(g)==CO2(g)����H= -395.4kJ/mol��C(s��ʯī��+O2(g)==CO2(g)����H= -393.5kJ/mol��
��ʯī�ͽ��ʯ��ȣ�ʯī���ȶ���______���ʯ���ȶ��ԡ�
��ʯī��C-C������______���ʯ��C-C�����ܡ�������������������С������������������
��2����֪H��H���ļ���Ϊ436 kJ��mol��1��Cl��Cl���ļ���Ϊ243 kJ��mol��1��H��Cl���ļ���Ϊ431 kJ��mol��1����H2(g) ��Cl2(g)=2HCl(g)�ķ�Ӧ��Ϊ______��
��3����֪���з�Ӧ�ķ�Ӧ�ȣ�
CH4(g)��H2O(g)��CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
CH4(g)��CO2(g)��2CO(g)��2H2(g) ��H2��-247.4 kJ��mol��1
��CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ______________��
���𰸡����� ���� ��H = -183kJ��mol-1 CH4(g)��2H2O(g)��CO2(g)��4H2(g) ��H��+659.8kJ��mol��1
��������
��1�����ʾ��е�����Խ��Խ�ȶ���
��2�����ݻ�ѧ��Ӧ�оɼ��Ķ������ȣ��¼����γɷ��ȣ�
��3�����ݸ�˹������⣻
��1������֪���ʯ��ʯīȼ��������ͬ�Ķ�����̼ʱ�ͷŵ������࣬����ͬ���Ľ��ʯ���е���������ʯī�����е�����������Խ��Խ�ȶ���ʯī���ȶ���
������Խ�ȶ��������Խ��Խ�ѷ�����Ӧ��ʯī�Ƚ��ʯ�ȶ�������ʯī�е�̼̼���Ƚ��ʯ�еļ��ܴ�
��2����ѧ��Ӧ�оɼ��Ķ������ȣ��¼����γɷ��ȣ���436 kJ/mol+243 kJ/mol-431 kJ/mol![]() 2=-183kJ/mol�����Ȼ�ѧ����ʽΪH2(g) ��Cl2(g)=2HCl(g) ��H = -183kJ/mol��
2=-183kJ/mol�����Ȼ�ѧ����ʽΪH2(g) ��Cl2(g)=2HCl(g) ��H = -183kJ/mol��
��3�����ݸ�˹���ɿ�֪����![]() 2-�ڿɵ�CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H��+659.8kJ/mol��
2-�ڿɵ�CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H��+659.8kJ/mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�