��Ŀ����
����Ŀ��ƽ�������ʾ�����������в��������ķϲ���(��SiO2��Fe2O3��CeO2��FeO������)��ijС���Դ˷ϲ���Ϊԭ�ϣ�������¹������̶���Դ���л��գ��õ�Ce(OH)4��
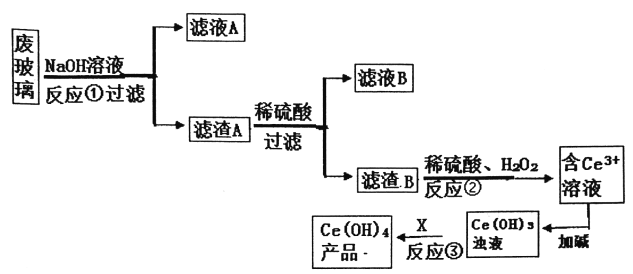
��֪:CeO2������ǿ���ǿ�Ce3+��ˮ�⣬���������£�Ce4+��ǿ�����ԡ�
(1)�ϲ�����NaOH��Һ��ϴǰ����Ҫ���еIJ���________����Ӧ�ٵ����ӷ���ʽ_______��
(2)��Ӧ�ڵ����ӷ�������____________��
(3)Ϊ�˵õ��ϴ���Ce3+��Һ����Ӧ��֮ǰҪ���еIJ�����______��
(4)��Ӧ����Ҫ������Լ�X������_________��
(5)�õζ����ⶨ�Ƶõ�Ce(OH)4��Ʒ���ȡ�
![]()
��FeSO4��Һ�ζ���_____��ָʾ�����ζ��յ������_______������FeSO4��Һ�ڿ�����¶��һ��ʱ����ٽ����еζ������ø�Ce(OH)4��Ʒ����������____(�ƫ����ƫС������Ӱ�족)��
���𰸡� ���� SiO2+2OH-=SiO32-+H2O 2CeO2+H2O2+6H+=2Ce3++O2��+4H2O ϴ�� O2������������ K3[Fe(CN)6] ���һ����Һʱ�����ɵ���ɫ����������Ҳ������ʧ ƫ��
���������ϲ�����ĩ(��SiO2��Fe2O3��CeO2��FeO������)������������Һ�������������������������ɹ����ƣ�Fe2O3��CeO2��FeO���ܣ����ˣ��õ���ҺA����Ҫ�ɷ�Ϊ�����ƣ�����A�ijɷ���Fe2O3��CeO2��FeO������A(Fe2O3��CeO2��FeO)��ϡ�������˵���ҺB�������������������Ļ����Һ������B�ijɷ���CeO2��CeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��O2����ӦΪ��2CeO2+H2O2+6H+=2Ce3++O2��+4H2O��Ce3+�Ӽ�����Ce(OH)3����Һ��Ce(OH)3����Һ����������Ce(OH)4��
(1)�ϲ�����NaOH��Һ��ϴǰ����Ҫ��Ҫ���飬������߽�ȡ�ʺͽ�ȡ���ʣ���Ӧ���ж����������������������ɹ����ƣ���Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O���ʴ�Ϊ�����飻SiO2+2OH-=SiO32-+H2O��
(2)��Ӧ��ΪCeO2��H2O2��ϡH2SO4��Ӧ����Ce3+��O2����ӦΪ��2CeO2+H2O2+6H+= 2Ce3++O2��+4H2O���ʴ�Ϊ��2CeO2+H2O2+6H+=2Ce3++O2��+4H2O��
(3)Ϊ�˵õ��ϴ���Ce3+��Һ����Ӧ��֮ǰ��Ҫ����B����ϴ�ӣ��ʴ�Ϊ��ϴ�ӣ�
(4)����������������Ӧ����Ce(OH)3����Һ����������Ce(OH)4����Ҫ������Լ�X������O2���ʴ�Ϊ��O2��
(5) K3[Fe(CN)6]�ܹ�������������Ӧ������������ɫ��������FeSO4��Һ�ζ�������K3[Fe(CN)6] ��ָʾ�����ζ��յ������Ϊ���һ����Һʱ�����ɵ���ɫ����������Ҳ������ʧ������FeSO4��Һ�ڿ�����¶��һ��ʱ����ٽ��еζ��������������ӱ��������������ӣ�����������Ũ�Ƚ��ͣ���������������Һ����������Բ�ø�Ce(OH)4��Ʒ����������ƫ�ʴ�Ϊ��K3[Fe(CN)6]�����һ����Һʱ�����ɵ���ɫ����������Ҳ������ʧ��ƫ��

 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�����Ŀ���о�NOx��CO�ȴ�����Ⱦ����Ĵ������Ա�����������Ҫ�����塣�ش��������⣺
��1��NOx��CO��Ӧ��������Ⱦ���������Ȼ�ѧ����ʽ����:��NO2(g)+CO(g)![]() CO2(g)+NO(g) ��H1=234.0 kJ��mol1����N2(g) +O2(g)
CO2(g)+NO(g) ��H1=234.0 kJ��mol1����N2(g) +O2(g)![]() 2NO(g)��H2=+179.5 kJ��mol1����2NO(g)+O2(g)
2NO(g)��H2=+179.5 kJ��mol1����2NO(g)+O2(g)![]() 2NO2(g) ��H3=112.3 kJ��mol1����Ӧ2NO2(g) +4CO(g)
2NO2(g) ��H3=112.3 kJ��mol1����Ӧ2NO2(g) +4CO(g)![]() N2(g)+4CO2(g)����H=__________ kJ��mol1��
N2(g)+4CO2(g)����H=__________ kJ��mol1��
��2����һ���¶��£���2 L�ĺ����ܱ������г���4.0 mol NO2��4.0 mol CO���ڴ��������·�����Ӧ��2NO2(g)+4CO(g)![]() N2(g) +4CO2(g)���������������£�
N2(g) +4CO2(g)���������������£�
0 min | 5 min | 10 min | 15 min | 20 min | |
c(NO2)/mol��L1 | 2.0 | 1.7 | 1.56 | 1.5 | 1.5 |
c(N2)/mol��L1 | 0 | 0.15 | 0.22 | 0.25 | 0.25 |
����0~10 min����CO2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ______________��
��Ϊʹ�÷�Ӧ�ķ�Ӧ�����������д�ʩ�пɲ��õ���________(����ĸ����)��
a����Сѹǿ b���ʵ������¶� c������CO��Ũ�� d��ѡ���Ч����
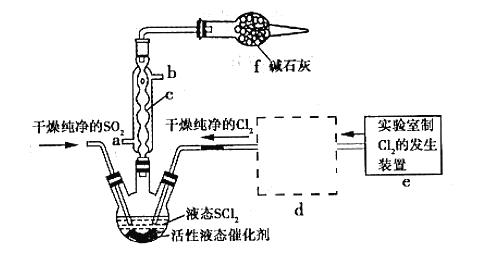
��3�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ���,��ô������������ͼװ�õ���������Һ����ȡ������������������������ء�
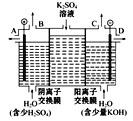
���õ��۵�������ӦʽΪ_______����ʱͨ�������ӽ���Ĥ��������____(����ڡ���С�ڡ����ڡ�)ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ���____(�A����B����C����D��)������
�������Ƶõ�����������������������Һ���Ϊ����ȼ�ϵ��,���ظ����ĵ缫��ӦʽΪ_____��
����Ŀ����������(CaO2)���������Ӽ������������������������Ϊ��ɫ�Ĺ��壬����ˮ���Ҳ������Ҵ������Ѻͼ�����Һ���������ᡣ
ijʵ��С����̽��CaO2�����ʼ���ʵ�����Ʒ���
��1��ʵ��̽��CaO2����ķ�Ӧ��
���� | ���� |
��ʢ��4 g CaO2�Ĵ��Թ��м���10mLϡ�������Һa | ���ҷ�Ӧ��������ʹ������ľ����ȼ������ |
ȡ5 mL��Һa���Թ��У���������ʯ����Һ | ��Һ��죬һ��ʱ�����Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
��CaO2�����ᷴӦ�Ļ�ѧ����ʽΪ_______________________________��
�ڼ���ʯ����Һ����Һ��ɫ��������Ϊ��Һa�д��ڽ϶��_________��
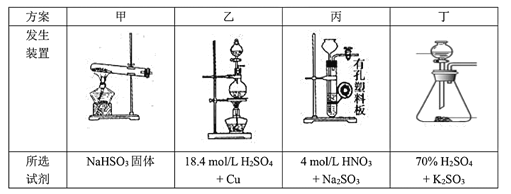
��2������ͼ��ʾװ���Ʊ��������ƣ��䷴Ӧԭ��ΪCa+O2![]() CaO2��
CaO2��
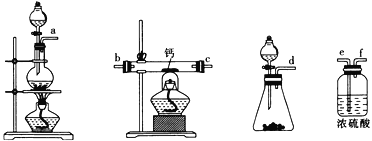
����ѡ��ʵ������Ҫ��װ�ã��������������ӵ�˳��Ϊ______________________���������ӿڵ���ĸ������װ�ÿɲ�ѡ��Ҳ���ظ�ʹ�ã���
�ڸ���������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£�����װ�õ������Ժ�װ��ҩƷ����Һ©��������ͨ������һ��ʱ�䣬����ҩƷ����Ӧ������_________________________________������������������װ�ã�ȡ�����
��3�����÷�Ӧ��Ca2++ H2O2+2NH3+8H2O=CaO2��8H2O��+2NH4+�ڼ��Ի�������ȡCaO2��װ����ͼ��ʾ��
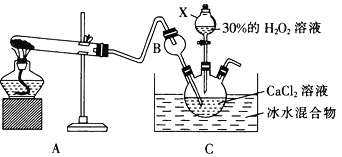
��NH3��Ca2+��H2O2�ķ�Ӧ�����������������______________________��
�ڷ�Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2��8H2O��������Ҫ�IJ���������_________������������ϴ�ӵIJ���Ϊ_____________________________________________��
��4�����ʵ��֤��CaO2�������Ա�FeCl3��������ǿ��____________________________��