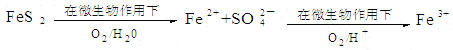��Ŀ����
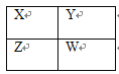
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺

��1��T��ԭ�ӽṹʾ��ͼΪ__��QԪ�����ڿ���ѧ��һ��ͬλ�صķ���Ϊ__��T���ӵİ뾶__������>������<������=��������������ý���Ԫ�ص����Ӱ뾶��
��2��Ԫ��Q������������Ӧˮ�����__�ԣ���������������������������__������ǿ����������������W������������Ӧˮ�����Ԫ�صķǽ����ԣ�Q__W������ǿ������������������
��3��R���⻯�����ʽΪ__����ˮ��Һ��__�ԣ��������������������������ԣ���ʵ���ҳ������ֹ��干����ȡ�����仯ѧ����ʽΪ__��
��4��ԭ��������R��1��Ԫ�ص�һ���⻯�������ĺ˻��������������__���������ӻ��������������ۻ������������õ���ʽ��ʾ���γɹ���__��
��5��R�ж���������������������Է���������С����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ������εĻ�ѧʽ��___��
���𰸡�![]() 14C < �� ���� ����
14C < �� ���� ���� ![]() ��
�� ![]() ���ۻ����� 2H+2
���ۻ����� 2H+2![]() ��
��![]() NaNO2
NaNO2
��������
��ͼ��֪��T���ڵ������ڣ����T��������������������������ȣ���֪��TΪAl��Al�ϱ���B��B�ұ���C��C�ұ���N������Q��R�ֱ�Ϊ��C��N��N�ұ���O��O�±���S������WΪS������������QΪC��RΪN��TΪAl��WΪS��
��1��Al��13��Ԫ�أ�����ԭ�ӽṹʾ��ͼΪ![]() ��14C������ͬλ��ʾ�ٷ������ţ�����������ý���Ԫ��ΪNa�������Ӻ������ӵ��Ӳ�����ͬ��������������С���뾶���ʴ�Ϊ��
��14C������ͬλ��ʾ�ٷ������ţ�����������ý���Ԫ��ΪNa�������Ӻ������ӵ��Ӳ�����ͬ��������������С���뾶���ʴ�Ϊ��![]() ��14C��<��
��14C��<��
��2��C������������Ӧˮ����Ϊ̼�ᣬ̼�������ᣬ�����ԣ�S������������Ӧˮ����Ϊ���ᣬ������ǿ�ᣬ�����ԣ�̼��������������ᣬ����������Ӧ��ˮ��������Խǿ���ǽ�����Խǿ������Q�ķǽ���������W���ʴ�Ϊ������ڣ����ڣ�
��3��N���⻯��ΪNH3�������ĵ���ʽΪ��![]() ��������ˮ��Ӧ���ɰ�ˮ����ˮ�Լ��ԣ�ʵ������ȡ������ԭ��Ϊ��
��������ˮ��Ӧ���ɰ�ˮ����ˮ�Լ��ԣ�ʵ������ȡ������ԭ��Ϊ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ���
���![]() ��
��
��4��ԭ��������R��1��Ԫ��ΪO��O���ĺ��⻯���ǹ������⣬��������ֻ�����ۼ������ڹ��ۻ�������γɹ��̿ɱ�ʾΪ��2H+2![]() ��
��![]() ���ʴ�Ϊ�����ۻ����2H+2
���ʴ�Ϊ�����ۻ����2H+2![]() ��
��![]() ��
��
��5��N����Է���������С��������ΪNO��NO��O2�������Ϊ4:1ǡ�ñ���ȫ���գ���Ӧ��NO��O2�����ʵ���֮��=4:1����Ӧ��O2�������������ϼ۴�0�۽���Ϊ-2�ۣ�1molO2��4mol���ӣ����ݵ��ӵ�ʧ�غ㣬4molNOʧȥ4mol���ӣ������Ļ�NԪ�ػ��ϼ�ֻ������1�����Բ�����NԪ�ػ��ϼ�Ϊ+3�ۣ�NԪ����+3�ۣ��京�������ΪNO2-�����Ըú�������ΪNaNO2���ʴ�Ϊ��NaNO2��

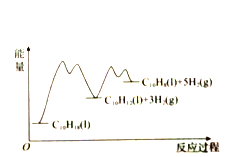
����Ŀ��Һ����Ϊһ��DZ�ڵ��������ȼ�ϣ����ڰ�ȫ�ԡ��۸�ȷ���ϻ�ʯȼ�Ϻ���ȼ�����Žϴ�����ơ���ش��������⣺
��.��֪��ӦN2(g)+3H2(g)![]() 2NH3(g)�Ļ��Ea1=akJ/mol����ػ�ѧ�������������£�
2NH3(g)�Ļ��Ea1=akJ/mol����ػ�ѧ�������������£�
��ѧ�� | H-H | N��N | N-H |
����/kJ��mol-1 | 436 | 946 | 391 |
(1)��Ӧ2NH3(g)![]() N2(g)+3H2(g)�Ļ��Ea2=______kJ/mol(�ú�a�Ĵ���ʽ��ʾ)��
N2(g)+3H2(g)�Ļ��Ea2=______kJ/mol(�ú�a�Ĵ���ʽ��ʾ)��
(2)��֪��
�� 4NH3(g)+3O2(g)��2N2(g)+6H2O(l) ��H1
�� 4NH3(g)+5O2(g)��4NO(g)+6H2O(l) ��H2
�� 4NH3(g)+6NO(g)��5N2(g)+6H2O(l) ��H3
����H1����H2����H3����֮��Ĺ�ϵΪ����H3= ______________ ��
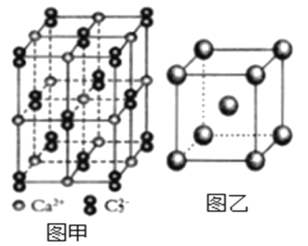
��.��������ȼ�ϵ��ԭ���о������°��ĺϳɣ���ع���ʱMV2+/MV+�ڵ缫��ø֮�䴫�ݵ��ӣ�����ԭ����ͼ��ʾ��
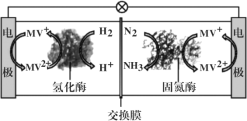
(3)�����ĵ缫��ӦʽΪ_______________��
(4)����·��ͨ��3mol����ʱ���ɲ������������(�����)Ϊ_______L����������n(H+)______(������������������������������)��
(5)��װ���ڸ����²�������������ԭ����_____��
����Ŀ����ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
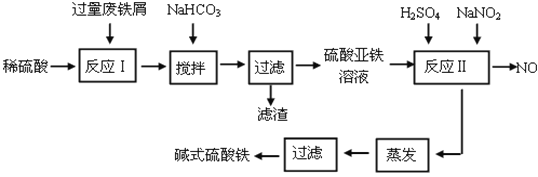
��֪������������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
��ʼ���� | 2.3 | 7.5 | 3.4 |
��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1����������NaHCO3��Ŀ���ǵ���pH��_____________________��Χ�ڡ�
��2����Ӧ���м���NaNO2��Ŀ���������������ӣ�д���÷�Ӧ�����ӷ���ʽΪ_____________________________________________________________��
��3����ʽ����������ˮ�����ɵ�Fe(OH)2+���ӿɲ���ˮ������Fe2(OH)42+ �ۺ����ӣ���ˮ�ⷴӦ�����ӷ���ʽΪ_________________________________��
��4����ҽҩ�ϳ����������������ᡢ����Ļ��Һ��Ӧ�Ʊ���ʽ�������������ҹ�����������Ʒ�в��ú���Fe2+��NO3����Ϊ�������ò�Ʒ���Ƿ���Fe2+��Ӧʹ�õ��Լ�Ϊ_________��
A����ˮ B��KSCN��Һ C��NaOH��Һ D������KMnO4��Һ
��5��Ϊ�ⶨ��Fe2+��Fe3+��Һ����Ԫ�ص��ܺ�����ʵ��������£�ȷ��ȡ20.00mL��Һ�ڴ�����ƿ�У���������H2O2������pH<2�����ȳ�ȥ����H2O2���������KI��ַ�Ӧ��,���� 0.1000 mol��L-1 Na2S2O3����Һ�ζ����յ㣬���ı���Һ20.00mL��
��֪��2Fe3++2I-=2Fe2++I2 2S2O32-+I2=2I-+S4O62-
����Һ����Ԫ�ص��ܺ���Ϊ_________g��L-1�����ζ�ǰ��Һ��H2O2û�г��������ⶨ����Ԫ�صĺ�������_______ (����ƫ���� ��ƫ���� ��������)