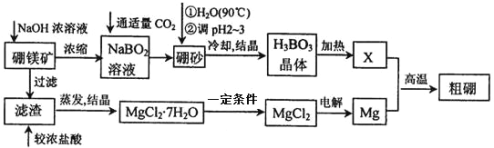
��Ŀ����
����Ŀ����ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�

��֪������������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
��ʼ���� | 2.3 | 7.5 | 3.4 |
��ȫ���� | 3.2 | 9.7 | 4.4 |
�ش��������⣺
��1����������NaHCO3��Ŀ���ǵ���pH��_____________________��Χ�ڡ�
��2����Ӧ���м���NaNO2��Ŀ���������������ӣ�д���÷�Ӧ�����ӷ���ʽΪ_____________________________________________________________��
��3����ʽ����������ˮ�����ɵ�Fe(OH)2+���ӿɲ���ˮ������Fe2(OH)42+ �ۺ����ӣ���ˮ�ⷴӦ�����ӷ���ʽΪ_________________________________��
��4����ҽҩ�ϳ����������������ᡢ����Ļ��Һ��Ӧ�Ʊ���ʽ�������������ҹ�����������Ʒ�в��ú���Fe2+��NO3����Ϊ�������ò�Ʒ���Ƿ���Fe2+��Ӧʹ�õ��Լ�Ϊ_________��
A����ˮ B��KSCN��Һ C��NaOH��Һ D������KMnO4��Һ
��5��Ϊ�ⶨ��Fe2+��Fe3+��Һ����Ԫ�ص��ܺ�����ʵ��������£�ȷ��ȡ20.00mL��Һ�ڴ�����ƿ�У���������H2O2������pH<2�����ȳ�ȥ����H2O2���������KI��ַ�Ӧ��,���� 0.1000 mol��L-1 Na2S2O3����Һ�ζ����յ㣬���ı���Һ20.00mL��
��֪��2Fe3++2I-=2Fe2++I2 2S2O32-+I2=2I-+S4O62-
����Һ����Ԫ�ص��ܺ���Ϊ_________g��L-1�����ζ�ǰ��Һ��H2O2û�г��������ⶨ����Ԫ�صĺ�������_______ (����ƫ���� ��ƫ���� ��������)
���𰸡�4.4~7.5 2H+ + Fe2++ NO2- = Fe3+ + NO�� + H2O 2Fe(OH)2+ +2H2O![]() Fe2(OH)42+ + 2H+ D 5.6 ƫ��
Fe2(OH)42+ + 2H+ D 5.6 ƫ��
��������
��1���Ʊ������������������������룬Ӧ������ҺpH����Al��OH��3����������Fe��OH��2��������Ӧ������ҺpH��4.4��7.5֮�䣻
��2��NaNO2��������������Ϊ�����ӣ�������ΪNO�������ӷ���ʽΪ2H+ + Fe2++ NO2- = Fe3+ + NO�� + H2O��
��3��[Fe��OH��]2+���ӣ��ɲ���ˮ������[Fe2��OH��4]2+�ۺ����ӣ����ݵ���غ�������غ��д����Ӧ�����ӷ���ʽΪ2[Fe��OH��]2++2H2O![]() [Fe2��OH��4]2++2H+��
[Fe2��OH��4]2++2H+��
��5���������ò�Ʒ���Ƿ���Fe2+����ʹ������KMnO4��Һ�����������Ը��������Һ��ɫ��
��6������������Ӧ�ɵù�ϵʽFe3+~S2O32-������n(Fe3+)=n(Na2S2O3)=0.1000mol/L��0.020L=0.0020mol����Һ����Ԫ�ص��ܺ���Ϊ0.0020mol��56g/mol��0.020L=5.6g/L�����ζ�ǰ��Һ��H2O2û�г�������H2O2Ҳ������I-�õ�I2�����ĵ�Na2S2O3��ƫ�࣬�������ⶨ����Ԫ�صĺ���ƫ�ߡ�

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϡ���CO2���������ȡ��ϩ��2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H<0
C2H4(g)+4H2O(g) ��H<0

(1)���÷�Ӧ��ϵ�������淴Ӧ���̱仯��ϵ��ͼ��ʾ����÷�Ӧ����H=_________kJ��mol1��(�ú�a��b��ʽ�ӱ�ʾ)
(2)���ֻ�ѧ���ļ��������ʾ��ʵ����������Ӧ����H= 152 kJ��mol1������е�x=___________��
��ѧ�� | C=O | H-H | C=C | C-H | O-H |
����/(kJ��mol-1) | 803 | 436 | x | 414 | 463 |
(3)��1 L�����ܱ�������ͨ��1 mol CO2��n mol H2����һ�������·���������Ӧ�����CO2��ת������(CO2)�뷴Ӧ�¶ȡ�Ͷ�ϱ�X[ n(H2)/n(CO2 )]�Ĺ�ϵ��ͼ��ʾ��

��X1_________(����>������<������=������ͬ)X2��
��ƽ�ⳣ��KA_______KB��KB________KC��
����B��ʱX=3����ƽ�ⳣ��KB=_____________(���������г���ʽ����)��
�����д�ʩ��ͬʱ��������Ӧ���ʺ����CO2ת���ʵ�����__________��
a�������¶� b��������� c������Ͷ�ϱ�X d��������ӷ�Ӧ��ϵ�з������

(4)��ϡ����Ϊ�������Һ������̫���ܵ�ؽ�CO2ת��Ϊ��ϩ�Ĺ���ԭ����ͼ��ʾ����N���ϵĵ缫��ӦʽΪ��_______________���õ��������������ܷ�Ӧ�Ļ�ѧ����ʽΪ��__________________��