ЬтФПФкШн
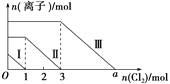
ЁОЬтФПЁПФћУЪЯЉ(НсЙЙШчЭМ1ЫљЪО)ЪЧЪГЦЗаавЕжаГЃгУЕФвЛжжЯуОЋЃЌФћУЪЯЉШлЕуЮЊ-74ЃЎ3ЁцЃЌЗаЕуЮЊ177ЁцЃЌФбШмгкЫЎЃЌдкНгНќ100ЁцЪБгавЛЖЈЕФЛгЗЂадЁЃФћУЪЁЂГШзгКЭшжзгЕШЫЎЙћЙћЦЄжаДцдкНЯЖрЕФФћУЪЯЉЃЌЪЕбщЪвЭЈГЃгУЫЎеєЦјеєСѓ(зАжУШчЭМ2ЫљЪО)ЕФЗНЗЈНЋФћУЪЯЉДгЙћЦЄжаЬсШЁГіРДВЂНјааКѓајЕФЗжРыЬсДПЁЃ
ЫЎеєЦјеєСѓЃКНЋ60gГШзгЦЄМєГЩЫщЦЌЭЖШывЧЦїBжаЃЌМгШы30mLеєСѓЫЎЃЌДђПЊTаЮЙмЛюШћЃЌЕуШМОЦОЋЕЦМгШШЫЎеєЦјЗЂЩњзАжУЃЌД§TаЮЙмжЇЙмПкгаДѓСПЫЎеєЦјУАГіЪБЙиБеЛюШћВЂПЊЪМЭЈШыРфФ§ЫЎЃЌЕБDжаЪеМЏЕНдМ60ЁЋ70mLСѓГівКЪБЭЃжЙеєСѓЁЃ
ЗжРыЬсДПЃКНЋСѓГівКЕЙШыЗжвКТЉЖЗЃЌУПДЮгУ10mLЖўТШМзЭщ(ЗаЕу39ЃЎ95Ёц)нЭШЁШ§ДЮЃЌнЭШЁвККЯВЂКѓжУгкзЖаЮЦПжаЃЌМгШыЪЪСПЮоЫЎСђЫсУОЃЌАыаЁЪБКѓЙ§ТЫЃЌНЋТЫвКНјааеєСѓГ§ШЅЖўТШМзЭщЃЌеєСѓЩеЦПжаЕУЕНжївЊГЩЗжЪЧФћУЪЯЉЕФГШгЭЁЃ
ЧыЛиД№ЯрЙиЮЪЬтЁЃ

ЃЈ1ЃЉвЧЦїAЕФУћГЦЪЧ____ЁЃАВШЋЙмЯТЖЫВЛФмЕжзЁвЧЦїAЕФЕзВПЃЌдвђЪЧ__ЁЃвЧЦїBжаЫЎеєЦјЕМШыЙмЙмПкНгНќЦПЕзЃЌФПЕФЪЧ___ЁЃ
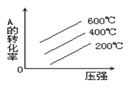
ЃЈ2ЃЉжБаЮРфФ§ЙмЬюФмЗёИќЛЛЮЊЧђаЮРфФ§Йм___(ЁАФмЁБЛђЁАЗёЁБ)ЃЌдвђЪЧ___ЁЃ
ЃЈ3ЃЉЗжРыЬсДПЙ§ГЬжаМгШыЮоЫЎСђЫсУОЕФзїгУЪЧ____ЁЃЖдТЫвКНјааеєСѓЪБКЯЪЪЕФМгШШЗНЗЈЪЧ____ЁЃ
ЁОД№АИЁПдВЕзЩеЦП ШєАВШЋЙмЯТЖЫЕжзЁAЕФЕзВПЃЌЫЎВЛФмНјШыЕМЙмДгЖјЮоЗЈЦНКтбЙЧП гаРћгкЫЎеєЦјгыФћУЪЯЉГфЗжНгДЅЃЌЪЙЦфБЛГфЗжеєГі Зё ШєЛЛГЩЧђаЮРфФ§ЙмЛсгаВПЗжСѓЗжВаСєдкРфФ§ЙмжаВЛРћгкЪеМЏ ИЩдя ЫЎдЁМгШШ
ЁОНтЮіЁП
ЃЈ1ЃЉвЧЦїAЕФУћГЦЪЧдВЕзЩеЦПЃЌАВШЋЙмЕФзїгУЪЧЦНКтДѓЦјбЙЧПЃЌШєЯТЖЫВЛФмЕжзЁвЧЦїAЕФЕзВПЃЌAжаЕФгавКЬхВЛФмНјШыЕМЙмДгЖјЮоЗЈЦНКтбЙЧПЁЃвЧЦїBжаЫЎеєЦјЕМШыЙмЙмПкНгНќЦПЕзЃЌФПЕФЪЧгаРћгкЫЎеєЦјгыФћУЪЯЉГфЗжНгДЅЃЌЪЙЦфБЛГфЗжеєГіЃЛ
Д№АИЮЊЃКдВЕзЩеЦПЃЛШєАВШЋЙмЯТЖЫЕжзЁAЕФЕзВПЃЌЫЎВЛФмНјШыЕМЙмДгЖјЮоЗЈЦНКтбЙЧПЃЛгаРћгкЫЎеєЦјгыФћУЪЯЉГфЗжНгДЅЃЌЪЙЦфБЛГфЗжеєГіЃЛ
ЃЈ2ЃЉШєЪЙгУЧђаЮРфФ§ЙмЛсгаВПЗжСѓЗжВаСєдкРфФ§ЙмжаВЛРћгкЪеМЏЁЃ
Д№АИЮЊЃКЗёЃЛШєЪЙгУЧђаЮРфФ§ЙмЛсгаВПЗжСѓЗжВаСєдкРфФ§ЙмжаВЛРћгкЪеМЏЃЛ
ЃЈ3ЃЉгУЫЎеєЦјеєСѓЕФЗНЗЈЬсШЁФћУЪЯЉЃЌ100ЁцЪБгаЫЎеєЦјЃЌЪЙжЦШЁЕФФћУЪЯЉжаКЌгаЫЎЃЌЕМжТжЦШЁЗжРыВЛДПЃЌМгШывЛЖЈЮоЫЎСђЫсУОПЩНЋФћУЪЯЉКЌгаЕФЫЎеєЦјЮќЪеЃЛЮЊСЫЪЙФћУЪЯЉЬсШЁИќДПОЛЃЌВЛаЏДјГіИќЖрЕФЫЎеєЦјЃЌПЩЪЙгУЮТЖШНЯЕЭЕФЫЎдЁМгШШЕФЗНЗЈЁЃ
Д№АИЮЊЃКИЩдяЃЛЫЎдЁМгШШЃЛ

 ПкЫуаЁзДдЊПкЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
ПкЫуаЁзДдЊПкЫуЫйЫуЬьЬьСЗЯЕСаД№АИЁОЬтФПЁПPbI2ЃЈССЛЦЩЋЗлФЉЃЉЪЧЩњВњаТаЭУєЛЏЬЋбєФмЕчГиЕФУєЛЏМСЁЊЁЊМзАЗЧІЕтЕФдСЯЁЃКЯГЩPbI2ЕФЪЕбщСїГЬШчЭМ1ЃК
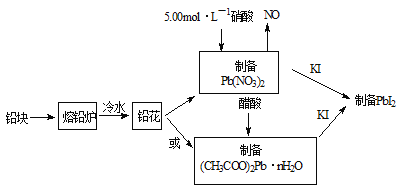

ЃЈ1ЃЉНЋЧІПщжЦГЩЧІЛЈЕФФПЕФЪЧ_____________ЁЃ
ЃЈ2ЃЉ31.05gЧІЛЈгУ5.00molЁЄLЃ1ЕФЯѕЫсШмНтЃЌжСЩйашЯћКФ5.00 molЁЄLЃ1ЯѕЫс_______mLЁЃ
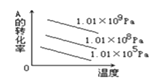
ЃЈ3ЃЉШЁвЛЖЈжЪСП(CH3COO)2PbЁЄnH2OбљЦЗдкN2ЦјЗежаМгШШЃЌВтЕУбљЦЗЙЬЬхВаСєТЪЃЉЃЈ![]() ЃЉЫцЮТЖШЕФБфЛЏШчЭМ2ЫљЪОЃЈвбжЊЃКбљЦЗдк75ЁцЪБвбЭъШЋЪЇШЅНсОЇЫЎЃЉЁЃ
ЃЉЫцЮТЖШЕФБфЛЏШчЭМ2ЫљЪОЃЈвбжЊЃКбљЦЗдк75ЁцЪБвбЭъШЋЪЇШЅНсОЇЫЎЃЉЁЃ
Ђй(CH3COO)2PbЁЄnH2OжаНсОЇЫЎЪ§ФПn=__________ЃЈЬюећЪ§ЃЉЁЃ
Ђк100ЁЋ200ЁцМфЗжНтВњЮяЮЊЧІЕФбѕЛЏЮяКЭвЛжжгаЛњЮяЃЌдђИУгаЛњЮяЮЊ___________ЃЈаДЗжзгЪНЃЉЁЃ
ЃЈ4ЃЉГЦШЁвЛЖЈжЪСПЕФPbI2ЙЬЬхЃЌгУеєСѓЫЎХфжЦГЩЪвЮТЪБЕФБЅКЭШмвКЃЌзМШЗвЦШЁ25.00mL PbI2БЅКЭШмвКЗжДЮМгШыбєРызгНЛЛЛЪїжЌRHжаЃЌЗЂЩњЃК2RH(s) + Pb2+(aq) = R2Pb(s) +2H+(aq)ЃЌгУзЖаЮЦПНгЪеСїГівКЃЌзюКѓгУеєСѓЫЎСмЯДЪїжЌжССїГівКГЪжаадЃЌНЋЯДЕгвККЯВЂЕНзЖаЮЦПжаЁЃМгШы2ЁЋ3ЕЮЗгЬЊШмвКЃЌгУ0.002500molЁЄLЃ1NaOHШмвКЕЮЖЈЃЌЕНЕЮЖЈжеЕуЪБгУШЅЧтбѕЛЏФЦБъзМШмвК20.00mLЁЃдђЪвЮТЪБPbI2ЕФKspЮЊ___________ЁЃ
ЃЈ5ЃЉЬНОПХЈЖШЖдЛЧЛЏЧІГСЕэШмНтЦНКтЕФгАЯьЁЃ
ИУЛЏбЇаЁзщИљОнЫљЬсЙЉЪдМСЩшМЦСНИіЪЕбщЃЌРДЫЕУїХЈЖШЖдГСЕэШмНтЦНКтЕФгАЯьЁЃ
ЬсЙЉЪдМСЃКNaIБЅКЭШмвКЁЂNaClБЅКЭШмвКЁЂFeCl3БЅКЭШмвКЁЂPbI2БЅКЭШмвКЁЂPbI2аќзЧвКЁЃ
аХЯЂЬсЪОЃКPb2+КЭClЃФмаЮГЩНЯЮШЖЈЕФPbCl42ЃТчРызгЁЃ
ЧыЬюаДЯТБэЕФПеАзДІЃК
ЪЕбщФкШн | ЪЕбщЗНЗЈ | ЪЕбщЯжЯѓМАдвђЗжЮі |
ЂйЛЧРызгХЈЖШдіДѓЖдЦНКтЕФгАЯь | ШЁPbI2БЅКЭШмвКЩйСПгквЛжЇЪдЙмжаЃЌдйЕЮШыМИЕЮNaIБЅКЭШмвК | ЯжЯѓЃКШмвКжаc(IЃ)діДѓЃЌЪЙQДѓгкСЫPbI2ЕФKsp |
ЂкЧІРызгХЈЖШМѕаЁЖдЦНКтЕФгАЯь | ________ | ЯжЯѓЃК________ двђЃК________ |
Ђл________ | дкPbI2аќзЧвКжаЕЮШыМИЕЮFeCl3БЅКЭШмвК | ЯжЯѓЃКЛЦЩЋЛызЧЯћЪЇ ________ |