��Ŀ����
1������������SnSO4����һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ��ij�о�С�����SnSO4�Ʊ�·�����£�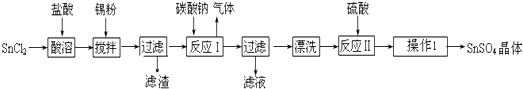
�������ϣ������������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ�������
��SnCl2��ˮ�����ɼ�ʽ�Ȼ�������Sn���ԭ������Ϊ119
�ش��������⣺
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ������ͬһ���壬��Ԫ�������ڱ��е�λ���ǵ������ڵڢ�A�壮
��2��������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
��3��SnCl2��ĩ���Ũ��������ܽ⣬����ƽ���ƶ�ԭ������ԭ��SnCl2ˮ�⣬����SnCl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣮
��4������Sn�۵��������������ٵ�����ҺpH �ڷ�ֹSn2+��������
��5�����������£�SnSO4����������˫��ˮȥ������������Ӧ�����ӷ���ʽ��Sn2++H2O2+2H+�TSn4++2H2O��
��6����С��ͨ�����з����ⶨ�������۵Ĵ��ȣ����ʲ����뷴Ӧ����
�ٽ��������������У������ķ�ӦΪ��Sn+2HCl�TSnCl2+H2����
�ڼ��������FeCl3��
������֪Ũ�ȵ�K2Cr2O7�ζ����ɵ�Fe2+�������ķ�ӦΪ��6FeCl2+K2Cr2O7+14HCl�T6FeCl3+2KCl+2CrCl3+7H2O
ȡ1.226g ���ۣ�������������Ӧ����ȥ0.100mol/L K2Cr2O7��Һ32.0ml����������������������93.2%��
���� SnCl2���������ܽ⣬�ټ������ۣ��ܽ�õ�SnCl2��Һ����̼���Ƴ��������ӣ����˵õ�����ϴ�Ӻ���������ܽ�õ���������Һ������Ũ����ȴ�ᾧ������ϴ�ӵõ����������壬
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ������ͬһ���壬���ڢ�A�壬����ԭ������������������Ԫ������ȷ�����ڵ����ڣ�
��2��������ͼ��֪���������Ǵ���Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ���
��3������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ��������������ᣬ����Sn2+ˮ�⣻
��4������Ϣ��֪��Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ��
��6�����ݵ���ת���غ��뷽��ʽ�ɵù�ϵʽSn��Sn2+��2Fe3+��2Fe2+��$\frac{1}{3}$K2Cr2O7���ݴ˼��㣮
��� �⣺SnCl2���������ܽ⣬�ټ������ۣ��ܽ�õ�SnCl2��Һ����̼���Ƴ��������ӣ����˵õ�����ϴ�Ӻ���������ܽ�õ���������Һ������Ũ����ȴ�ᾧ������ϴ�ӵõ����������壬
��1����Ԫ����̼Ԫ������ͬһ���壬���ڢ�A�壬ԭ�Ӻ˵����Ϊ50����50-2-8-8-18=14����Sn���ڵ������ڣ��������ڱ��е�λ��Ϊ���������ڵڢ�A�壬
�ʴ�Ϊ���������ڵڢ�A�壻
��2��������ͼ��֪���������Ǵ���Һ�еõ����ᾧˮ�ľ��壬ֻ�ܲ�ȡ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ���
�ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
��3������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��Sn Cl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣬
�ʴ�Ϊ��SnCl2ˮ�⣬����SnCl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
��4������Ϣ��֪��Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
�ʴ�Ϊ����ֹSn2+��������
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
�ʴ�Ϊ��Sn2++H2O2+2H+�TSn4++2H2O��
��6����������������������Ϊx����
Sn��Sn2+��2Fe3+��2Fe2+��$\frac{1}{3}$K2Cr2O7���㣮
119g $\frac{1}{3}$mol
1.226g��x 0.100mol/L��0.032L
��$\frac{119g}{1.226xg}$=$\frac{\frac{1}{3}mol}{0.100mol/L��0.032L}$�����x=93.2%��
�ʴ�Ϊ��93.2%��
���� ����SnSO4�Ʊ���֮��Ϊ���壬����ѧ���Թ������̵����⡢���ʵķ����ᴿ���Ķ���Ŀ��ȡ��Ϣ�����������û�ѧ������д���ζ�Ӧ�ü����ù�ϵʽ���еļ���ȣ��Ѷ��еȣ���ѧ���Ļ���֪ʶ���������нϸߵ�Ҫ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�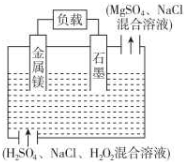 þ-������������һ�����͵ĸ����ܵ�Դ����ṹ��ͼ��ʾ������˵������ȷ���ǣ�������
þ-������������һ�����͵ĸ����ܵ�Դ����ṹ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | þ���õ�Դ������������ӦMg-2e-�TMg2+ | |
| B�� | �õ�ط�Ӧ����ʽ��Mg+H2O2+2H+�TMg2++2H2O | |
| C�� | �ŵ�ʱ��Һ�е�Cl-�������ƶ� | |
| D�� | �ŵ�ʱ������Χ��Һ��pH���� |
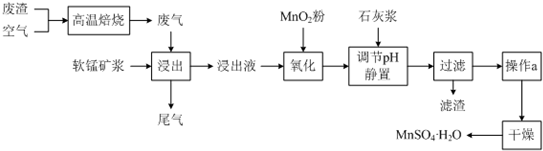
��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+�������������ӣ��йؽ��������γ������������ʱ��pH���±���
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
��1��д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ��SO2+MnO2=MnSO4��
��2��д��������������Ҫ��Ӧ�����ӷ���ʽ��2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��3�����������Һ���м���ʯ�ҽ�������pH��ֽ�ⶨ����pH��pHӦ���ڷ�Χ��4.7��8.3��
| A�� | 1.0 mol•L-1��KNO3��Һ�У�H+��Fe2+��Cl-��SO42- | |
| B�� | ʹ���ȱ��ɫ����Һ�У�Mg2+��Cu2+��SO42-��NO3- | |
| C�� | 0.1 mol•L-1��NaOH��Һ�У�Na+��NH4+��Cl-��HCO3- | |
| D�� | ˮ�����c��H+��=1��10-13mol•L-1����Һ�У�Ca2+��K+��ClO-��NO3- |
| A�� | 30mL | B�� | 70mL | C�� | 90mL | D�� | 140mL |
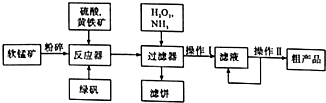 �����̿���Ϊ�������Ӽ��������ʣ�Ҳ������ijЩ��ѧ��Ӧ�Ĵ�����������ˮ���������Ҵ�����ҵ�ϳ������̿���Ҫ�ɷ�ΪMnO2������MgSO4�����ʣ��Ʊ������̣�����������£�
�����̿���Ϊ�������Ӽ��������ʣ�Ҳ������ijЩ��ѧ��Ӧ�Ĵ�����������ˮ���������Ҵ�����ҵ�ϳ������̿���Ҫ�ɷ�ΪMnO2������MgSO4�����ʣ��Ʊ������̣�����������£�