��Ŀ����
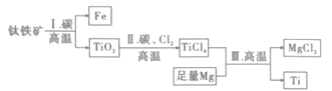
����Ŀ���й�������ĸ���������мƻ����в�����ǰ�ƽ������캽ĸ��Ҫ���������Ͳ��ϡ���ĸ������Ҫ�ͳ������ĸ�ļװ�Ҫ���£���ĸ�����Ҫ��ʴ��
��1�������ֿ���ʴ����ǿ��Ni2+��̬ԭ�ӵĺ�������Ų�Ϊ_______________________.
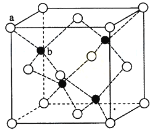
��2����ĸ�װ�Ϳ��һ�����µIJ��Ͼ۹�����ṹ��ͼ��ʾ������Cԭ���ӻ���ʽΪ______�ӻ������л�����Cԭ�Ӽ�����γ�˫������������Siԭ�Ӽ������γ�˫����ԭ����_____________.
��3��������Ԫ�ص�ҡ������ˮ�к��д���±��Ԫ�ء�
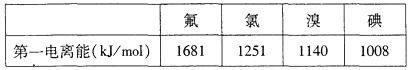
�������±������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________����Ԫ�ط��ţ�
�����ݼ۲���ӶԻ������ۣ�Ԥ��ClO�Ŀռ乹��Ϊ___________�Σ�д��һ��ClO�ĵȵ�����Ļ�ѧ����___________.
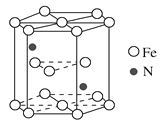
��4�������������������̲��Ŵ�������Դ�����й衢�����̡�п�ȡ�ij�ִ��Ե������ľ����ṹ��ͼ��ʾ���û�����Ļ�ѧʽΪ________________���������ױ߳�Ϊa nm����Ϊc nm�������ִ��Ե������ľ����ܶ�Ϊ________g��cm3(�ú�a��c��NA��ʽ�ӱ�ʾ)
���𰸡�1s22s22p63s23p63d8 sp3 Si��ԭ�Ӱ뾶�ϴ�ԭ�Ӽ��γɵ������ϳ���p-p����ص��̶Ⱥ�С�������γ����� I ���� SO![]() Fe3N
Fe3N 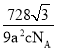 ��1021
��1021
��������
��Ni��̬ԭ�ӵĺ�������Ų�Ϊ1s22s22p63s23p63d84s2��Ni2+��̬ԭ�ӵĺ�������Ų�Ϊ1s22s22p63s23p63d8��
�ʴ�Ϊ1s22s22p63s23p63d8��
��Cԭ����Χ��������ԭ���γ�4����������C��û�йµ��Ӷԣ�����ӻ���ʽΪsp3�ӻ������л�����Cԭ�Ӽ�����γ�˫������������Siԭ�Ӽ������γ�˫����ԭ���ǣ�Si��ԭ�Ӱ뾶�ϴ�ԭ�Ӽ��γɵ������ϳ���p-p����ص��̶Ⱥ�С�������γ�������
�Ǣٵ�һ������ԽС��Խ��ʧȥ���ӣ���Խ���γ������ӣ�������п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����I��
�ʴ�ΪI��
��ClO3��������ԭ�ӵŵ��Ӷ���Ϊ![]() =1���������Ӷ���Ϊ3��VSEPRģ��Ϊ�������Σ��ռ乹��Ϊ�����Σ����������ClO3���ĵȵ�����Ļ�ѧ����ΪSO32����
=1���������Ӷ���Ϊ3��VSEPRģ��Ϊ�������Σ��ռ乹��Ϊ�����Σ����������ClO3���ĵȵ�����Ļ�ѧ����ΪSO32����
�ʴ�Ϊ������SO32����
���þ�̯���������к�Fe��![]() ��N��2���û�����Ļ�ѧʽΪFe3N������������Ϊ
��N��2���û�����Ļ�ѧʽΪFe3N������������Ϊ![]() g���������ױ߳�Ϊa nm����Ϊc nm�����������Ϊ
g���������ױ߳�Ϊa nm����Ϊc nm�����������Ϊ![]() ��a��10-7cm��
��a��10-7cm��![]() ��6��c��10-7cm=
��6��c��10-7cm=![]() a2c��10-21cm3�����ִ��Ե������ľ����ܶ�Ϊ
a2c��10-21cm3�����ִ��Ե������ľ����ܶ�Ϊ![]() g����
g����![]() a2c��10-21cm3��=
a2c��10-21cm3��=![]() ��1021g/cm3��
��1021g/cm3��
�ʴ�ΪFe3N��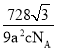 ��1021��
��1021��

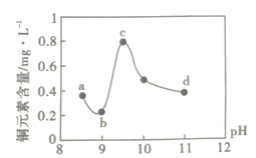
����Ŀ��ij��·��������ҵ��ˮ����������������ŷŵ���ˮ�����±���ʾ��Ϊ�о���ˮ��Cu2+���������pH��ȡ5�ݵ����ķ�ˮ���ֱ���30%��NaOH��Һ����pH��8.5��9��9.5��10��11�����ú����ϲ���Һ��ͭԪ�صĺ�����ʵ��������ͼ��ʾ���������ϣ�
ƽ���Cu(OH)2+4NH3![]() [Cu(NH3)4]2+2OH-
[Cu(NH3)4]2+2OH-
ƽ���Cu(OH)2+2OH-![]() [Cu(OH-)4]2-
[Cu(OH-)4]2-
��Ŀ | ��ˮˮ�� | �ŷű� |
pH | 1.0 | 6~9 |
Cu2+/mg��L-1 | 72 | ��0.5 |
Nh4+/mg��L-1 | 2632 | ��15 |
����˵��������ǣ� ��
A.a~b�η����ķ�ӦΪ��Cu2++2OH-=Cu(OH)2��
B.b~c�Σ���pH���ߣ�Cu(OH)2�������ӣ�����ƽ��������ƶ���ͭԪ�غ�������
C.c~d�Σ���pH���ߣ�c(OH-)���ӣ�ƽ��������ƶ���ͭԪ�غ����½�
D.d���Ժ���c(OH-)���ӣ�ͭԪ�غ�����������