��Ŀ����
4����ѧ���ѧ����������ᡢ����������أ�������������ȷ���ǣ�������| A�� | �����Ҵ����ͣ�����������һ�������Ҵ������������ܽ��ͻ�����β�����к������ŷ� | |
| B�� | ��ҵ����ʯ�����úȼ�պ��γɵ��������������������Ƶ�ʯ�� | |
| C�� | Ϊ����Ч�ķ�չ�����Դ�����õ��ˮ�ķ��������Ʊ�H2 | |
| D�� | ����ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� | |
| E�� | ���û�ѧ��Ӧԭ������ƺ������µ�ҩ�� |
���� A��ʹ���Ҵ������ܼ����к�������ŷţ��Ҵ��ǿ�������Դ��
B��ʯ�������������еĶ�����������������ƣ������������Եõ�������ƣ�
C�����ˮ�ķ��������Ʊ�H2 �����ĸ������Դ�������ã�
D��CO��NOx��Ӧ�ڴ��������·�Ӧ���ɵ����Ͷ�����̼�����壻
E�����û�ѧԭ�������Ʊ��µ����ʣ�
��� �⣺A��ʹ���Ҵ������ܼ����к�������ŷţ��Ҵ��ǿ�������Դ����A����
B��ʯ�������������еĶ�����������������ƣ������������Եõ�ʯ�࣬��B��ȷ��
C�����ˮ�ķ��������Ʊ�H2 �����ĸ������Դ�������ã����Բ����õ��ˮ�ķ��������Ʊ�H2����C����
D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ����������N2��CO2����D��ȷ��
E�����û�ѧԭ�������Ʊ��µ����ʣ��������û�ѧ��Ӧԭ������ƺ������µ�ҩ���E��ȷ��
��ѡ��BDE��
���� ��Դ�����ϡ���������Ϣ�ǵ��������Ĵ���Ҫ�����ܹ�ע��������Դ�ķ��ࡢ��ȱ�㡢Ӧ�ü��Ի�����Ӱ�����⣬�ڿ����г��ֵ�Ƶ�ȼ��ߣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
15��������һ����Ҫ��������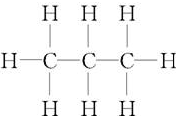 ��ʾ�䣨������
��ʾ�䣨������
 ��ʾ�䣨������
��ʾ�䣨������| A�� | �ṹ��ʽ | B�� | �ṹʽ | C�� | ����ʽ | D�� | ����ʽ |
9����ѡ���ʵ����Լ������Գ�ȥ�����ڵ����ʣ���д����صĻ�ѧ����ʽ
A��KSCN��Һ B��Fe C����ˮ D��ϡ���� E��NaOH��Һ F����ˮ
A��KSCN��Һ B��Fe C����ˮ D��ϡ���� E��NaOH��Һ F����ˮ
| ���� | �����Լ� | �йػ�ѧ����ʽ |
| FeCl2��FeCl3������Һ�� | Fe | 2FeCl3+Fe=3FeCl2 |
| MgO��Al2O3�� | NaOH��Һ | Al2O3+2NaOH�T2NaAlO2+H2O |
13��Na2O2��CaC2������ˮ��Ӧ���ҷ�Ӧ�ж�������ų�������˵����ȷ���ǣ�������
| A�� | ������������ԭ��Ӧ | B�� | ���ɵ������Ϊ�������� | ||
| C�� | ��Ӧ��ˮ���������� | D�� | ��Ӧ������ |
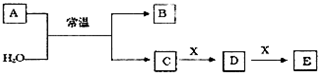
 ��
��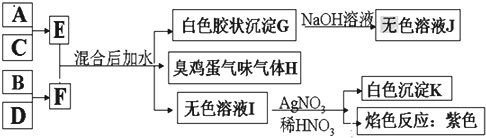
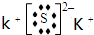 ��
��
 X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y$\stackrel{��}{��}$Z+W
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y$\stackrel{��}{��}$Z+W ��
�� ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W����Ԫ�ص�ԭ������֮��Ϊ34�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ�Y��Z�������ڣ�Y��Wλ��ͬ���壮
ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W����Ԫ�ص�ԭ������֮��Ϊ34�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ�Y��Z�������ڣ�Y��Wλ��ͬ���壮