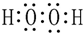��Ŀ����
14��A��B��C��D��E��X����ѧ�������������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ������֪A�ɶ����ڷǽ���Ԫ����ɣ�B����Ư�����ҹ����ֽ⣮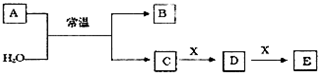
��AΪ����Ԫ���γɵĻ������E��ˮ��Ӧ����F��Ũ��Һ��C�а���G������
��1��A�ĵ���ʽΪ
 ��
����2��C��D�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO��+6H2O��
��3��ʵ���Ҽ���G�������ӵķ����ǣ�ȡG�������Թ��������Һ���μ�NaOH��Һ�����ȣ�����ʪ�ĺ�ɫɫʯ����ֽ�����Թܿڣ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ����ɫ��˵������NH4+��
���� A��B��C��D��E��X����ѧ���������A�ɶ����ڷǽ���Ԫ����ɣ�B����Ư�����ҹ����ֽ⣬��BΪHClO����AΪ����Ԫ���γɵĻ������E��ˮ��Ӧ����G��Ũ��Һ��C�а��̲�������CΪNH3�����BΪHClO������Ԫ���غ�ɼ����ϼ۵������֪AΪNCl3�����Ƶ�DΪNO��EΪNO2��XΪO2��FΪHNO3��GΪNH4NO3������ת����ϵ���ݴ˴��⣮
��� �⣺A��B��C��D��E��X����ѧ���������A�ɶ����ڷǽ���Ԫ����ɣ�B����Ư�����ҹ����ֽ⣬��BΪHClO����AΪ����Ԫ���γɵĻ������E��ˮ��Ӧ����G��Ũ��Һ��C�а��̲�������CΪNH3�����BΪHClO������Ԫ���غ�ɼ����ϼ۵������֪AΪNCl3�����Ƶ�DΪNO��EΪNO2��XΪO2��FΪHNO3��GΪNH4NO3������ת����ϵ��
��1��AΪNCl3��A�ĵ���ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��C��D�Ļ�ѧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO��+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO��+6H2O��
��3��GΪNH4NO3������������ΪNH4+�����鷽��Ϊ��ȡG�������Թ��������Һ���μ�NaOH��Һ�����ȣ�����ʪ�ĺ�ɫɫʯ����ֽ�����Թܿڣ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ����ɫ��˵������NH4+��
�ʴ�Ϊ��ȡG�������Թ��������Һ���μ�NaOH��Һ�����ȣ�����ʪ�ĺ�ɫɫʯ����ֽ�����Թܿڣ��д̼�����ζ������ų�����ʹ��ʪ�ĺ�ɫʯ����ֽ����ɫ��˵������NH4+��
���� ���⿼��������ƶϣ����ڲ²���֤����Ŀ������������ѧ���������������ͳ���˼ά�������Ѷ��еȣ�

| A�� | A��B��C��D | B�� | A��C��B��D | C�� | A��C��D��B | D�� | D��B��A��C |
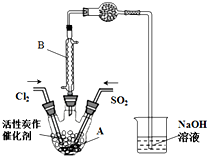 ijѧϰС�����ݷ�Ӧ��SO2��g��+Cl2��g��?SO2Cl2��g����H��0������Ʊ������ȣ�SO2Cl2����װ����ͼ���й���Ϣ�����ʾ��
ijѧϰС�����ݷ�Ӧ��SO2��g��+Cl2��g��?SO2Cl2��g����H��0������Ʊ������ȣ�SO2Cl2����װ����ͼ���й���Ϣ�����ʾ��| SO2Cl2 | Cl2 | SO2 | |
| �۵�/�� | -54.1 | -101 | -72.4 |
| �е�/�� | 69.1 | -34.6 | -10 |
| ���� | ��ˮ���� ����ˮ�� |
��2��B������������ʹ�ӷ��IJ���SO2Cl2����������
��3��Ϊ�˱��ڻ����ķ�������߷�Ӧ���ת���ʣ�Aװ�õķ�Ӧ�������ѡ��a��
a����ˮԡ b������ c��������69.1��
��4�����ͨ���Cl2��SO2����ˮ�����������Ͷ���������ܷ�����Ӧ�Ļ�ѧ����ʽΪSO2+Cl2+2H2O=H2SO4+2HCl��
��5��ʵ��ʱ��ͨ������Cl2��Aװ���еĿ������ߣ��ٻ���ͨ������SO2����������Ӧ����ַ�Ӧ����ͨ��Cl2ʹװ���е�SO2�����ձ��б����գ������������õ�SO2Cl2�м�ˮ�����ְ����������õõ���ɫ��ҺW��
�پ�����SO2Cl2��H2O��Ӧ���ڷ�������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽSO2Cl2+2H2O=H2SO4+2HCl��
����ɫ��ҺW�е������ӳ�������OH-�⣬�������������������ӣ�������ҺW�������������ӷ�����ȡ����W��Һ���Թ��У��������Ba��NO3��2��Һ���в�����ϡ����İ�ɫ����������˵����Һ�к���SO42-�����ˣ�����Һ�еμ�HNO3�ữ���ټ���AgNO3��Һ��������ɫ��������˵����Һ����Cl-��
�۷�Ӧ��ɺ���W��Һ���ձ��зֱ�μӹ�����BaCl2��Һ�������ְ�ɫ�������˳���������ϡ���ᣬ�����ˡ�ϴ�ӡ���������õ��Ĺ��������ֱ�ΪXg��Yg������SO2+Cl2?SO2Cl2��Ӧ�У�SO2��ת���ʣ��ú�X��Y�Ĵ���ʽ��ʾ����
| A�� | T��ʱ��pH=6�Ĵ�ˮ�У����е�OH-��ĿΪ1��10-6NA | |
| B�� | ��״���£�22.4LCCl4�к��еĹ��õ��Ӷ���ĿΪ4.0NA | |
| C�� | 50g98%��Ũ�����У���������ԭ����ĿΪ2NA | |
| D�� | 1molNa2O2������CO2��ַ�Ӧת�Ƶĵ�����ĿΪNA |
| A�� | Cu | B�� | K2SO4 | C�� | SO2 | D�� | NaOH��Һ |
| A�� | ʵ�����п���ȼ�շ�����CO��H2��H2S�ȿ�ȼ��β�� | |
| B�� | ������������茶������Ƿ��нᾧˮ��ȡһ�Թܣ���ҩ����2����������茶��壬�����Թܿ�����һ��պ��������ˮ����ͭ��ĩ������ͬʱ�������ӣ���ȼ�ƾ��Ƽ����Թܣ��۲����� | |
| C�� | ȡһҩ�����ۡ���ҩ��ʳ������һƬ���ϱ�Ĥ�ϣ���Ͼ��ȣ���һ�ι�ˮ���������ϱ�Ĥ�����������п������������������з��̸� | |
| D�� | ���к͵ζ�ʵ���У��ȿ��ñ���Һ�ζ�����Һ��Ҳ���ô���Һ�ζ�����Һ |
| A�� | �����Ҵ����ͣ�����������һ�������Ҵ������������ܽ��ͻ�����β�����к������ŷ� | |
| B�� | ��ҵ����ʯ�����úȼ�պ��γɵ��������������������Ƶ�ʯ�� | |
| C�� | Ϊ����Ч�ķ�չ�����Դ�����õ��ˮ�ķ��������Ʊ�H2 | |
| D�� | ����ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� | |
| E�� | ���û�ѧ��Ӧԭ������ƺ������µ�ҩ�� |
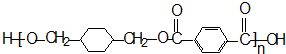
 -CH3+Cl2��
-CH3+Cl2�� -CH2Cl+HCl
-CH2Cl+HCl
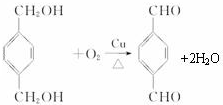
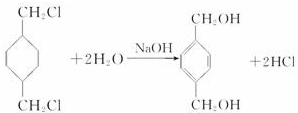
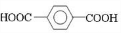 +
+ $\stackrel{һ��������}{��}$
$\stackrel{һ��������}{��}$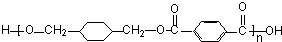 +��2n-1��H2O��
+��2n-1��H2O��