��Ŀ����
3����1���£�N2H4����NO2��һ��˫��ֻ���ƽ������������ʻ�Ϸ�����Ӧ����N2��H2O��g������֪8g��������������Ӧ�зų�142kJ���������Ȼ�ѧ����ʽΪ2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=-1136KL/mol����2��0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ���������Ȼ�ѧ��Ӧ����ʽΪB2H6��g��+3O2��g��=B2O3��g��+3H2O��l������H=-2165KJ/mol��
��֪H2O��l��?H2O��g������H=+44kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ���ų���������1016.5 kJ��
���� ��1������8g��ȼ�շ���142KJ�����1mol��ȼ�շ��ȶ��٣�����д�Ȼ�ѧ����ʽ����д�Ȼ�ѧ����ʽʱ��ע�����ʵ�״̬����H��ֵ�뻯ѧ�������Ķ�Ӧ��
��2�����Ⱦ�0.3mol������ȼ�շ���649.1KJ�������1mol������ȼ�շ��ȶ��٣�����д�Ȼ�ѧ����ʽ���ڶ����У�ע��11.2L������ȼ������ˮ�����ʵ�����
��� �⣨1��8gN2H4�����ʵ���Ϊ$\frac{8g}{32g•mo{l}^{-1}}$=0.25mol����1molN2H4��Ӧ�ų�������Ϊ$\frac{1mol}{0.25mol}$=568KJ�����Ȼ�ѧ����ʽΪ��
2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=-1136KL/mol���ʴ�Ϊ��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g����H=-1136KL/mol��
��2��0.3mol����̬������ȼ�շų�������Ϊ649.5KJ����1mol��̬������ȼ�շų�������Ϊ$\frac{1mol}{0.3mol}$=2165KJ�������Ȼ�ѧ����ʽΪ��
B2H6��g��+3O2��g��=B2O3��g��+3H2O��l������H=-2165KJ/mol����״����11.2L������Ϊ0.5mol����ȫȼ������1.5molҺ̬ˮ����2165KJ��2=1082.5KJ����֪H2O��l��?H2O��g������H=+44kJ/mol����1.5molҺ̬ˮ��Ϊˮ����������1.5mol��44KJ/mol=66KJ�������1082.5KJ-66KJ=1016.5KJ���ʴ�Ϊ��B2H6��g��+3O2��g��=B2O3��g��+3H2O��l������H=-2165KJ/mol��1016.5��
���� ���⿼�����Ȼ�ѧ����ʽ����д����ϸ�˹���ɣ�ע��ע�����ʵ�״̬����H��ֵ�뻯ѧ�������Ķ�Ӧ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �ж�һ��Ԫ���ǽ������Ƿǽ��� | B�� | �жϻ�������Ԫ�صĻ��ϼ� | ||
| C�� | �жϻ�ѧ������ | D�� | �жϻ�������ܽ�� |
| A�� | ����ǿ����H2SiO3��H2CO3��HNO3 | B�� | ԭ�Ӱ뾶��С��Na��S��O | ||
| C�� | ����ǿ����KOH��NaOH��LiOH | D�� | �ǽ�����ǿ����I��Cl��F |
��CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H=+49.0kJ•mol-1
��CH3OH��g��+$\frac{1}{2}$O2��g���TCO2��g��+2H2��g����H=-192.9kJ•mol-1
����˵����ȷ���ǣ�������
| A�� | CH3OH��ȼ����Ϊ192.9 kJ•mol-1 | |
| B�� | 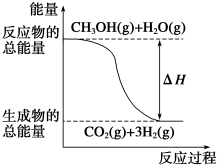 ��Ӧ���е������仯��ͼ��ʾ | |
| C�� | CH3OHת���H2�Ĺ���һ��Ҫ�������� | |
| D�� | ���ݢ���֪��ӦCH3OH��l��+$\frac{1}{2}$O2��g���TCO2��g��+2H2��g���ġ�H��-192.9 kJ•mol-1 |
| A�� | NH3��4��10���ӵķ��ӣ������Σ����л�ԭ�� | |
| B�� | NH3��������ˮ��������Ȫʵ�飻������Һ����Һ������������� | |
| C�� | �����Ƿǵ���ʣ���ˮ�ǵ���� | |
| D�� | �������е�ԭ������������Ϊ8 |
| A�� | ��ԭ�ԣ�F-��Cl-��Br-��I- | B�� | ���ԣ�NaOH��Mg��OH��2��Al��OH��3 | ||
| C�� | �⻯���ȶ��ԣ�H2S��HF��H2O | D�� | ���ԣ�HClO4��H3PO4��H2SO4 |
 �����Ӽ���������
�����Ӽ��������� X��Y��ZΪ���������ֵ��ʣ�Z����ɫֲ�������õIJ���֮һ��A��BΪ���������������һ�������¿ɷ�����ͼ��ʾ�ķ�Ӧ�������ڷ���Һ�н��еķ�Ӧ����
X��Y��ZΪ���������ֵ��ʣ�Z����ɫֲ�������õIJ���֮һ��A��BΪ���������������һ�������¿ɷ�����ͼ��ʾ�ķ�Ӧ�������ڷ���Һ�н��еķ�Ӧ���� ��
��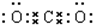 ��
��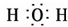 ��
��