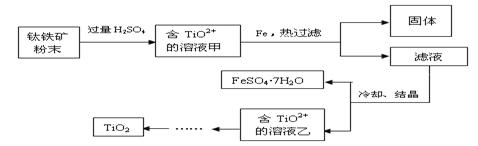
��Ŀ����
������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ����������ҵ�ϳ�����NaClO�������������������������£�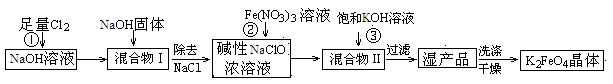
��Ҫ��ӦΪ��3NaClO��2Fe(NO3)3��10NaOH �� 2Na2FeO4����3NaCl��6NaNO3��5H2O
Na2FeO4��2KOH��K2FeO4��2NaOH��
��1��д����Ӧ�ٵ����ӷ���ʽ�� ��
��2���ӡ������II���з����K2FeO4���и���Ʒ�����Ƕ�����Ҫ�Ļ�����Ʒ������һ���ڹ�ҵ�������ȼҵԭ�ϵ������� ��
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣ͼ��Ϊ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻ͼ��Ϊһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣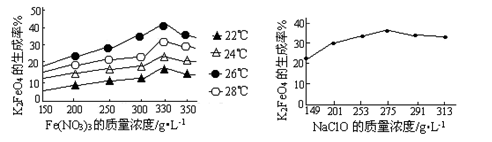
ͼ�� ͼ��
������ͼ��������ҵ����������¶�Ϊ �棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ ��
��1��Cl2��2OH�� ��Cl����ClO����H2O ��2��NaCl ��3���� 26 1.2
���������������1���������������壬�ܺ��ռ���Һ��Ӧ����Ӧ�����ӷ���ʽΪCl2��2OH�� ��Cl����ClO����H2O��
��2����������ȷ������������Ϊ�Ȼ��ơ����������Լ��������ƵĻ���������Ӧ��֮�����û����II�������NaNO3��NaCl��NaOH��K2FeO4�����з����K2FeO4�õ��ĸ���Ʒ��NaNO3��NaCl��NaOH������NaNO3��ըҩ��NaCl������ζƷ���ȼҵԭ�ϵȡ�
��3��Ѱ������¶�Ҫ�߱������������¶��·�Ӧ���ʿ죬���ɸ�����صIJ��ʽϴ������棬���Ը���ͼ���֪��ҵ����������¶�Ϊ26�棬��Ϊ�ڸ��¶������ɸ�����صIJ������ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ��1.2��
���㣺���鳣��Ԫ�صĵ��ʼ��仯������ۺ�Ӧ���Լ�ͼ�������Ӧ�õ�

 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�����ѧ��ѡ��2����ѧ�뼼������15�֣�
��̬��ҵ���Ľ��裬�����������ֻ��������Ҫ����ѭ���������ۺͳ�ֿ��Ǿ��õĿɳ�����չ��������ij��ҵ��Ƶ����ᣭ��泥�ˮ����������ˮ����ˮ���ã��Σ��ȣ�������������̬��ҵ������ͼ��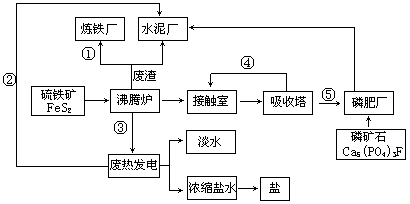
����������ҵ���̻ش��������⣺
��1����ԭ�ϡ���Դ����ͨ�Ƕȿ��Ǹ���ҵӦ���ڣ�������
����
| A������ɽ������ | B���غ��������� | C��������С��� | D��������½ |
��3������¯������Ӧ�Ļ�ѧ����ʽ�������������������������������� ,�ʳ��IJ�Ʒ���ոƣ�����Ҫ�ɷ��������������� (�ѧʽ)��
��4���ȵ糧����ȴˮ��������������������Ũ����ˮ����ȡ���������ȡ��������������д��һ�ּ��ɣ���
��5��������̬�����������������¯�������������������������ܵ��������������������������������������������������������� ����д�����㣩��
����ĽṹΪCH3��CH2��COOH,�������ǰ�ȫ��Ч�ķ�ù��������,һ���Լ�ʽ̼��пΪԭ�ϵ�����������������: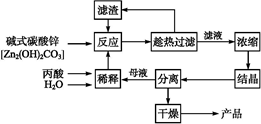
| ��� | n(����)�� n(��ʽ̼��п) | ��Ӧ�¶�/�� | ����п����/% |
| 1 | 1��0.25 | 60 | 67.2 |
| 2 | 1��0.25 | 80 | 83.5 |
| 3 | 1��0.25 | 100 | 81.4 |
| 4 | 1��0.31 | 60 | 89.2 |
| 5 | 1��0.31 | 80 | 90.1 |
| 6 | 1��0.31 | 100 | 88.8 |
(1)̽����ʵ������ѹ�������(���ϱ�):��Ӧʱ��2 h,��ˮ��45 g,n(����)��n(��ʽ̼��п)=1��
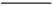 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 (2)�����ղ��á���·ѭ������ʽ,�������Ʊ����ռ�㡢���ʸ���,���������������������������ŵ㡣
(3)ij��ʵ��ʱ,��37.0 g��������220 mLˮ��,�����������������Ż����������Ʊ�,���յñ���п49.6 g,��ô�ʵ�����п�IJ���Ϊ��������(д���������)��
��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO�����ʣ���ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪��ˮ������Һ�д���Na2CrO4��NaAlO2��Na2ZnO2������
��1��ˮ�������Һ��____�ԣ����ᡱ����������С�����
��2������������չ���������Na2CrO4�Ļ�ѧ����ʽ��
____Cr(OH)3+____Na2CO3+_____  = ____Na2CrO4+___CO2+_____
= ____Na2CrO4+___CO2+_____
��3������II����Ҫ�ɷ���Zn(OH)2��___________________________________��
��4����ϵ�в�������Ϊ����������H2SO4��________��ȴ�ᾧ�����ˡ���������H2SO4Ŀ����________________________��
��֪���ٳ�ȥ����II����Һ�д������·�Ӧ��2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� ��ѧʽ | 20�� | 60�� | 100�� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
��5����ҵ�ϻ�������ˮ�����˺����Һ��Na2CrO4����������H2SO4����ʯī���缫���������������д�����ɸ��ĵ缫��Ӧ����ʽ____________________________��
���������LiFePO4��һ��������������ӵ�صĵ缫���ϡ�ij�����������졢﮻�ʯLiAl��SiO3��2��������Ca2+��Mg2+���Σ���̼�۵�ԭ����������������ﮡ�����Ҫ�����������£�
��֪��2LiAl��SiO3��2 + H2SO4(Ũ) 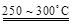 Li2SO4 + Al2O3��4SiO2��H2O��
Li2SO4 + Al2O3��4SiO2��H2O��
| �¶�/�� | 20 | 40 | 60 | 80 |
| �ܽ��(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
| �ܽ��(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
��1�����������пɷ����Al2O3������ͼ��ʾ����д�����ɳ��������ӷ���ʽ ��
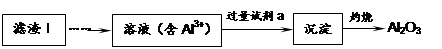
��2�����������Ҫ�ɷ��ǣ� ���ѧʽ����
��3������Һ���м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ����
��
��4��д���ڸ�����������������﮵Ļ�ѧ����ʽ ��
��5����������﮵���ܷ�ӦΪ��FePO4+Li
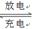 LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ ��
LiFePO4������еĹ������ʿɴ���Li������д���õ�طŵ�ʱ��������Ӧ�� �����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ �� �������ʵ��Ʊ������Ϲ�ҵ����ʵ�ʵ���( )
| A��������ͨ�����ʯ��ˮ����Ư�� |
| B�������ӽ���Ĥ����ⱥ��ʳ��ˮ�Ʊ��ռ���������� |
| C����������������Ϻ��ȼ��������ˮ�����Ʊ����� |
| D����SO2��O2�Ļ�����Ӹ�ѹ��ͨ���Ӵ��ң��Ʊ�SO3 |