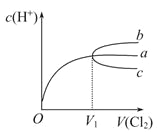
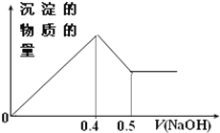
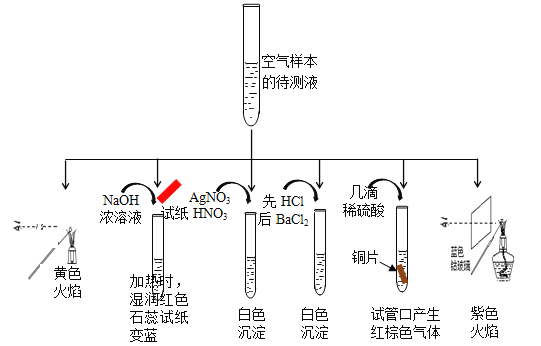
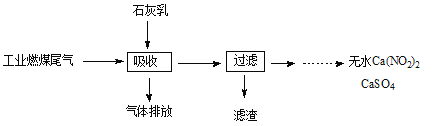
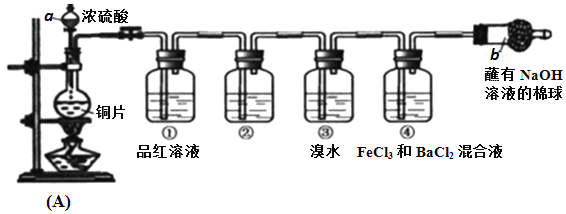
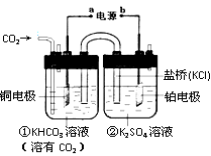
��Ŀ����
����Ŀ����������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3(s) + 3H2(g)![]() W (s) + 3H2O (g)����ش��������⣺
W (s) + 3H2O (g)����ش��������⣺
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ____________________��
��2�� ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ___________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ ��Ӧ��������������������������
��3����H2��ԭWO2Ҳ�ɵõ������١���֪��
WO2(s) + 2H2(g)![]() W (s) + 2H2O (g) ��H =" +66.0" kJ��mol�C1
W (s) + 2H2O (g) ��H =" +66.0" kJ��mol�C1
WO2(g) + 2H2(g)![]() W (s) + 2H2O (g) ��H =��137.9 kJ��mol�C1
W (s) + 2H2O (g) ��H =��137.9 kJ��mol�C1
��WO2(s)![]() WO2(g) ����H = ______________________��
WO2(g) ����H = ______________________��
��4����˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2(g)![]() WI4(g)������˵����ȷ����________������ţ���
WI4(g)������˵����ȷ����________������ţ���
a���ƹ��ڵ�I2��ѭ��ʹ�� b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
���𰸡���10�֣���1��K= c3(H2O)/c3(H2)��2��60% ����
��3��+203.9 kJ/mol��4��a��b
�������������������1����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ�����Ը��ݷ�Ӧ�Ļ�ѧ����ʽ��֪���÷�Ӧ��ƽ�ⳣ������ʽK= c3(H2O)/c3(H2)��
��2�����ݻ�ѧ����ʽWO3(s) + 3H2(g)![]() W (s) + 3H2O (g)��������ˮ������ϵ����ͬ��������ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����Ӧ�����������Ϊ3��������ͬ�����£���������ʵ��������ȣ���H2��ƽ��ת����Ϊ3mol��5mol��100%=60%����Ӧ��ƽ��ʱ�����¶ȵ����ߣ� H2��ˮ����������ȼ�С����˵�������¶�ƽ������Ӧ�����ƶ�����������Ӧ�����ȷ�Ӧ��
W (s) + 3H2O (g)��������ˮ������ϵ����ͬ��������ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����Ӧ�����������Ϊ3��������ͬ�����£���������ʵ��������ȣ���H2��ƽ��ת����Ϊ3mol��5mol��100%=60%����Ӧ��ƽ��ʱ�����¶ȵ����ߣ� H2��ˮ����������ȼ�С����˵�������¶�ƽ������Ӧ�����ƶ�����������Ӧ�����ȷ�Ӧ��
��3�����ݸ�˹���ɿ�֪�����������õ�WO2��s��![]() WO2��g����������H����66kJ/mol��137.9kJ/mol����203.9kJ/mol��
WO2��g����������H����66kJ/mol��137.9kJ/mol����203.9kJ/mol��
��4���÷�Ӧ�ڲ�ͬ�¶��£���Ӧ���еķ����Dz�ͬ�ġ����������ɵ��ʵ⣬���¶Ƚ��ͺ������ĵ⣬���Եƹ��ڵ�I2��ѭ��ʹ�ã�a����ȷ���÷���ʽ��֪��WI4�ڵ�˿�Ϸֽ⣬������W�ǹ��壬������ڵ�˿�ϣ�b��ȷ��c���������¶ȣ���Ӧ���ʶ�������d����ѡab��

����Ŀ������ˮ�к�����ᵼ�����ж���ˮ�����ܽ������Ҫ��As( ��)�������κ�As(V)��������ʽ���ڡ�
(1)������Ϊͬһ����Ԫ�أ���ԭ�ӽṹʾ��ͼΪ___________________��
(2)����Ԫ�������ɣ�����˵����ȷ����____________________(����ĸ����)��
a.����������Ӧˮ��������ԣ�S>P>As b. ԭ�Ӱ뾶��S>P>As c.�ǽ����ԣ�S>P>As
(3)���ڵ���ˮ�������Դ�ж��ּ��裬����һ����Ϊ�Ǹ�����Ļ�����(FeS2)������ΪFe(OH)3��ͬʱ����SO42-���������������������ˮ��FeS2��O2���������ӷ���ʽΪ_________________________��
(4)ȥ��ˮ���е��飬���Ƚ�As(�� )ת��ΪAs(V)��ѡ��NaClO��ʵ�ָ�ת����
��֪��Ͷ��ǰˮ��pH=5.81��0.1 mol/L NaClO��ҺpH=10.5����Һ�����������õ������Ǵ����ᡣ
�о�NaClOͶ������As(��)�����ʵ�Ӱ��õ����½����
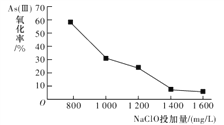
�����˽����ԭ����_________________________��
(5)ǿ�����ӽ�����������������������ʽ���ڵ�As(V)���Ӷ��ﵽȥ��As��Ŀ�ġ�
��֪��һ�������£�As(V)�Ĵ�����ʽ���±���ʾ��
pH | <2 | 2��7 | 7��11 | 11��14 |
������ʽ | H3AsO4 | H2AsO4- | HAsO42- | HAsO42-��AsO43- |
pH=6ʱ��NaClO����������(H3AsO3)�����ӷ���ʽ��_____________________��