��Ŀ����
18���Ҵ��������г������л���ܽ�����ͼ��ʾ�Ķ��ַ�Ӧ��A��B��C��D�����л��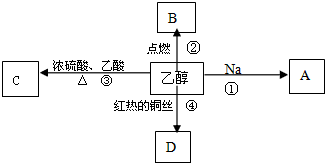
��1���Ҵ������й����ŵ��������ǻ���
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�٣�2Na+2CH3CH2OH2CH3CH2ONa+H2����
��Ӧ�ۣ�CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O����Ӧ�����У�Ũ��������ã���������ˮ����
��Ӧ�ܣ�2CH3CH2OH+O2$��_{��}^{����}$2CH3CHO+2H2O���ڷ�Ӧ�����У�ͭ˿�������䣨���С�����䣩��
��3�������ʵ�����CH4��C2H6��C2H60��C6H6�������ʣ�����ͬ״������ȫȼ��ʱ����O2����������C6H6��������������4�����ʣ�����ͬ״������ȫȼ��ʱ����O2����������CH4������CO2����������C6H6�����ѧʽ��
��4����ҵ�����Ҵ��ķ���ʽΪCH2=CH2+H2O$��_{��}^{����}$CH3CH2OH��
���� ���Ҵ���ת����ϵ��֪���Ҵ���������������DΪCH3CHO��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CΪCH3COOCH2CH3���Ҵ����Ʒ�����Ӧ����AΪCH3CH2ONa���Ҵ�ȼ�����ɶ�����̼���Դ˽����⣮
��� �⣺���Ҵ���ת����ϵ��֪���Ҵ���������������DΪCH3CHO��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CΪCH3COOCH2CH3���Ҵ����Ʒ�����Ӧ����AΪCH3CH2ONa���Ҵ�ȼ�����ɶ�����̼��
��1���Ҵ����еĹ�����Ϊ�ǻ����ʴ�Ϊ���ǻ���
��2����Ӧ��Ϊ2CH3CH2OH+2Na��2CH3CH2ONa+H2����
��Ӧ��Ϊ���ᡢ�Ҵ���������Ӧ������ʽΪCH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O����Ӧ����������ˮ�������ã�
��Ӧ�ܵķ���ʽΪ2CH3CH2OH+O2$��_{��}^{����}$2CH3CHO+2H2O����Ӧ��ͭ���������ã��������䣬
�ʴ�Ϊ����2Na+2CH3CH2OH2CH3CH2ONa+H2������CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O����������ˮ������2CH3CH2OH+O2$��_{��}^{����}$2CH3CHO+2H2O�����䣻
��3���л���ȼ�յ�ͨʽΪCxHyOz+��x+$\frac{y}{4}$_$\frac{z}{2}$��O2��xCO2+$\frac{y}{2}$H2O��
���趼Ϊ1mol�����������������ʵ����ֱ�Ϊ2mol��3.5mol��3mol��7.5mol��
������������4�����ʣ�������Խ�����ĵ�����Խ�࣬ӦΪ���飬���ڱ��ĺ�̼����������ɶ�����̼��࣬
�ʴ�Ϊ��C6H6�� CH4��C6H6��
��4����ҵ�����Ҵ��ķ���ʽΪCH2=CH2+H2O$��_{��}^{����}$CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O$��_{��}^{����}$CH3CH2OH��
���� ���⿼���л����ƶϣ��漰ϩ��������ȩ�����ᡢ����������ת�����ѶȲ��������ڻ���֪ʶ�Ĺ��̣�

| A�� |  ��Ӧ������������ϵ������ͼ��ʾ | |
| B�� | �����÷�Ӧ��Ƴ�ԭ�����пΪ���� | |
| C�� | ��H��ֵ�뷴Ӧ����ʽ�Ļ�ѧ�������й� | |
| D�� | ���������Ϊԭ��أ�����32.5gп�ܽ�ʱ��ת�Ƶ�����Ϊ2NA |
| A�� | ͬϵ��Ļ�ѧ�������� | |
| B�� | ͬλ�صĻ�ѧ���ʼ�����ͬ | |
| C�� | ��Է���������ͬ�Ļ��������ͬ���칹�� | |
| D�� | ͬ��������֮���ת����ͬ���칹��֮���ת�������ڻ�ѧ�仯 |
| A�� | �����������������仯 | |
| B�� | ʯ���ѽ���Եõ�����ϩ | |
| C�� | ��֬ˮ��ɵõ���������� | |
| D�� | ���ۺ���ά�ص���ɶ��ǣ�C6H10O5��n��ˮ�����ղ��ﶼ�������� |
��1��������������˵��������Ӧ�Ѵ�ƽ�����AC��
A�������������ƽ��Ħ���������ֲ���
B��2v��H2����=v��CH3OH����
C�������������ѹǿ���ֲ���
D����λʱ��������nmol CO��ͬʱ����2nmol H2
��2�����ݻ��̶��ĺ����ܱ������г���CO��H2����������Ӧ����Ӧ�ڵ�4minʱ��ﵽ���ȣ���ʱ������ѹǿ�뷴Ӧǰ֮��Ϊ3��5�����������ʵĸ������ʵ���Ũ�����±���
| ʱ��/Ũ�� | c��CO����mol/L�� | c��H2����mol/L�� | c��CH3OH����mol/L�� |
| ��ʼ | 0.200 | 0.300 | 0.000 |
| ��4min | a | b | c |
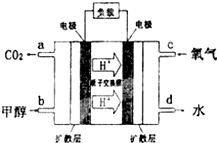
��3���״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ����� Ⱦ�綯�����ij��ص�أ��乤��ԭ����ͼ��ʾ����ȼ�ϵ�صĵ�ط�ӦʽΪ2CH3OH��g��+3O2��g���T2CO2��g��+4H2O��l�������ĵ缫��ӦʽΪCH3OH-6e-+H2O=CO2+6H+������õ�ع���ʱ��·��ͨ��1.2mol���ӣ�������CH3OH��0.2mol��
| A�� | ���ȶ��ԣ�HCl��H2S��PH3 | B�� | ԭ�Ӱ뾶��Na��Mg��Cl | ||
| C�� | ����ǿ����H2SiO3��H2CO3��H2SO4 | D�� | ����ǿ����LiOH��NaOH��KOH |
| A�� | HCl��HNO3�ų�H2һ���� | B�� | �����зų�H2��� | ||
| C�� | HCl�зų�H2��� | D�� | HNO3�ȴ���ų�H2�� |
| A�� | ����ϩ��ͬ���칹�壨����˳���칹������5�� | |
| B�� | �ױ��ܱ����Ը����������Ϊ�����ᣬ�����鲻��ʹ���Ը��������ɫ��˵���л�������еĻ��ż�����Ӱ�� | |
| C�� | ���Ӵ�����ͼ��ʾ��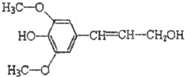 �ܷ���������ȡ����ˮ�⡢�Ӿ۷�Ӧ �ܷ���������ȡ����ˮ�⡢�Ӿ۷�Ӧ | |
| D�� | ������ij���ʵ���Һ�μӵ����Ƶ�������Һ�У�ˮԡ���Ⱥ����������ɣ�������һ������ȩ�� |
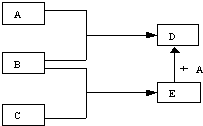 ��һ���������ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�
��һ���������ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�