��Ŀ����
����CO��H2�ڴ����������ºϳɼ״�����Ӧ���£�CO(g)+2H2(g)=CH3OH(g)����2L�ܱ������г������ʵ���֮��Ϊ1��2��CO��H2���ڴ��������³�ַ�Ӧ�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯����ͼ��ʾ������˵����ȷ����

A���÷�Ӧ�ġ�H<O����p1��p2��p3
B����C��ʱ��H2ת����Ϊ75%
C����Ӧ���ʣ�v��(״̬A��>v��(״̬B��
D���ں��º�ѹ�����£�����ܱ��������ٳ���1molCH3OH��
��ƽ��ʱCH3OH�������������
��ϰ��ϵ�д�
�����Ŀ
20�����н�����ʵ�ķ���ʽ��ȷ���ǣ�������
| A�� | ���ȿ���ǿ������Һȥ������CO32-+2H2O?H2CO3+2OH- | |
| B�� | �����������ˮ������Ӧ��ȡ����������3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2 | |
| C�� | ���ռ���Һ��ϴ�����������Ĥ��2OH-+Al2O3=2AlO2-+H2O | |
| D�� | ��ϡ����ϴ������������Ӧ���Թܣ�3Ag+4H++NO3-=3Ag++NO��++2H2O |
18�����ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ������ʾ��
����������ȷ���ǣ�������
| Ԫ�ش��� | X | Y | Z | W |
| ԭ�Ӱ뾶/pm | 160 | 143 | 70 | 66 |
| ��Ҫ���ϼ� | +2 | +3 | +5��+3��-3 | -2 |
| A�� | X��YԪ�صĽ����ԣ�X��Y | |
| B�� | һ�������£�Z������W�ij�������ֱ������ZW2 | |
| C�� | һ�������£�W���ʿ��Խ�Z���ʴ����⻯�����û����� | |
| D�� | Y������������Ӧ��ˮ����������ϡ��ˮ |
13����֪�������ƾƾ��ķ�Ӧ�ֱ�Ϊ����C6H10O5��n �����ۣ�+nH2O $\stackrel{����}{��}$nC6H10O6 �������ǣ���C6H10O6 $\stackrel{����}{��}$2CH3CH2OH+2CO2 ����ij������2t�ĺ�����54%�������ƾƾ�������ڷ���������85%�ĵ���ת��Ϊ�Ҵ������Ƶõľƾ��к�5%��ˮ������Ƶ������ľƾ���������
| A�� | 0.85t | B�� | 0.65t | C�� | 1.55t | D�� | 0.55t |
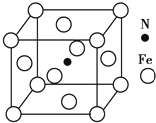
12����Li��Al��Si���ɵ�ij��Ԫ�������̬����ṹ��ͼ��ʾ������˵����ȷ���ǣ�������

| A�� | �û����ﻯѧʽ�ɱ�ʾΪLiAlSi | |
| B�� | ������Al��Li����CsCl�Ǽ� | |
| C�� | ������Al��Si���ɽ��ʯ�Ǽ� | |
| D�� | ��������ÿ��Al���������LiΪ4�� |


 �����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯����������
�����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯���������� ��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2��
��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2��
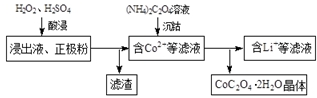
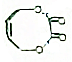 ��д����ϳ�·��ͼ��
��д����ϳ�·��ͼ��