��Ŀ����
1��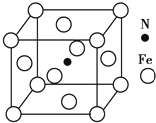 �����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯����������
�����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯������������1��N��ԭ�ӽṹʾ��ͼΪ
 ��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2��
��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2����2��H��N��O�ĵ縺�Դ�С�����˳����H��N��O��
��3����NO2+��Ϊ�ȵ������һ�ַ���ΪN2O���ѧʽ����������-NH2���е�ԭ�ӵ��ӻ�����Ϊsp3��
��4��1mol�������к��ЦҼ������ʵ���Ϊ12mol��
��5��Fe��N�γɵ�ij�����ᄃ����ͼ��ʾ����þ���Ļ�ѧʽΪFe4N��
���� ��1��N��ԭ��������7����������ԭ������Ϊ26������������ԭ����д��̬ԭ�ӵĵ����Ų�ʽ��
��2�����ݵ縺�Եı仯���ɱȽϵ縺�Դ�С��
��3��NO2+�ĵ�����Ϊ22����ȵ�����ΪN2O����CO2��CS2�ȣ���������-NH2���е�ԭ���γ�3���ļ���һ���¶Ե��ӣ��۲���Ӷ���Ϊ4����ԭ��Ϊsp3�ӻ���
��4����������̼̼֮�京��һ���Ҽ�����6����̼��֮�京��һ���Ҽ�����6��������1mol�������к��ЦҼ�����ĿΪ12mol��
��5���ڸþ����У�����Fe��8��$\frac{1}{8}$+2��$\frac{1}{2}$=4��NΪ1������þ���Ļ�ѧʽΪFe4N��
��� �⣺��1��N��ԭ��������7��ԭ�ӽṹʾ��ͼΪ ������ԭ������Ϊ26�������������ԭ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2���ʴ�Ϊ��
������ԭ������Ϊ26�������������ԭ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2���ʴ�Ϊ�� ��1s22s22p63s23p63d64s2��[Ar]3d64s2��
��1s22s22p63s23p63d64s2��[Ar]3d64s2��
��2����Ԫ�����ڱ���ͬһ���ڴ�����Ԫ�صĵ縺������ǿ��ͬһ������ϵ���Ԫ�صĵ縺����������֪�縺��ǿ��˳��ΪO��N��H��
�ʴ�Ϊ��H��N��O��
��3��NO2+�ĵ�����Ϊ22����ȵ�����ΪN2O����CO2��CS2�ȣ���������-NH2���е�ԭ���γ�3���ļ���һ���¶Ե��ӣ��۲���Ӷ���Ϊ4����ԭ��Ϊsp3�ӻ����ʴ�Ϊ��N2O����CO2��CS2�ȣ���sp3��
��4����������̼̼֮�京��һ���Ҽ�����6����̼��֮�京��һ���Ҽ�����6��������1mol�������к��ЦҼ�����ĿΪ12mol���ʴ�Ϊ��12 mol����12��6.02��1023����
��5���ڸþ����У�����Fe��8��$\frac{1}{8}$+2��$\frac{1}{2}$=4��NΪ1������þ���Ļ�ѧʽΪFe4N���ʴ�Ϊ��Fe4N��
���� ���⿼���Ϊ�ۺϣ��漰�����Ų�ʽ���縺�ԡ��ӻ���������Լ�����ṹ������֪ʶ����Ŀ����һ���Ѷȣ�����ע�⾧����λ�����жϷ�����


| A�� | ÿ�����ټ�����к���2������̼ԭ�� | |
| B�� | 1 mol���ټ��������1 mol Br2������Ӧ | |
| C�� | 1 mol���ټ��������4 mol H2������Ӧ | |
| D�� | ���ټ���������ᷴӦ������NaOH��Һ��Ӧ |
| A�� | ������Һ������������̼��īˮ���ɷ������������ | |
| B�� | ������ˮʱ�����˻�ѧ�������仯������ɱ�������������� | |
| C�� | ���ͷ����Ҫ�ɷ�������غ����ʣ��ֱ����ڵ���ʺͷǵ���� | |
| D�� | ��������Һ�м���CuSO4��Һ���г��������������ʿ����ڵ����ʵķ������ᴿ |
| A�� | 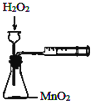 �����ⶨ��ѧ��Ӧ���� | B�� | 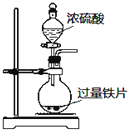 �����Ƭ��Ũ����ķ�Ӧ | ||
| C�� |  ��ʳ��ˮ����ȡNaCl | D�� | 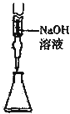 ��ȡ15.00mLNaOH |
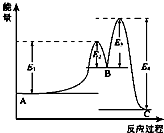 ij��Ӧ��������ӦA?B?C���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������
ij��Ӧ��������ӦA?B?C���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������| A�� | ������Ӧ��Ϊ���ȷ�Ӧ | B�� | ���������ı䷴Ӧ���ʱ� | ||
| C�� | ���ֻ�������C���ȶ� | D�� | ������Ӧ�С�H=E1-E4 |
| A�� | �������ʿ��û����ǽ������� | B�� | ��������Ԫ�ز�һ����ͬ����Ԫ�� | ||
| C�� | ���ʿ�ͬΪ��̬��Ҳ��ͬΪ��̬ | D�� | �����ڳ����½��з�Ӧ |
| A�� | ����KMnO4��Һ | B�� | ������ˮ | C�� | ϡ���� | D�� | �������� |
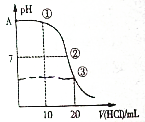 �����£���0.1000mol•L-1 HCl��Һ�ζ�20.00mL 0.1000mol•L-1 NH3•H2O��Һ���ζ�������ͼ������˵����ȷ���ǣ�������
�����£���0.1000mol•L-1 HCl��Һ�ζ�20.00mL 0.1000mol•L-1 NH3•H2O��Һ���ζ�������ͼ������˵����ȷ���ǣ�������| A�� | A���pH=13 | |
| B�� | ����Һ��c��NH4+����c��Cl-����c��OH-��=c��H+�� | |
| C�� | ����Һ����ʱ��Һ�е�ˮ�����c��H+����ԭ��ˮ�е����c��H+��С | |
| D�� | �ζ������п��ܳ��֣�c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+�� |
