��Ŀ����
��֪һ���¶��£�2X(g)��Y(g) 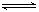 ?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����)
?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����)
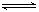 ?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����)
?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����)| A��m��4 | B��a��b | C��a< | D��m��2 |
D
b>2c��˵����������X��ת���ʶ�����������X��ת���ʡ���Ϊ��������X��Y�����ʵ�������Y������2������˵������ѹǿƽ����������Ӧ�����ƶ��ģ���m��3����Ϊ�ǿ��淴Ӧ��������2 mol X��1 mol Y��Ӧ���ﵽƽ��״̬ʱ���ų�������������a kJ����a��b������D��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
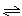 2SO3����֪V��SO2��==0.05mol��l-1��min-1����2min��SO3��Ũ��Ϊ�� ��
2SO3����֪V��SO2��==0.05mol��l-1��min-1����2min��SO3��Ũ��Ϊ�� �� 2C(g)��ƽ����¶Ȳ��䣬����ѹǿ��ƽ�������ƶ���ƽ�ⳣ��Kֵ����
2C(g)��ƽ����¶Ȳ��䣬����ѹǿ��ƽ�������ƶ���ƽ�ⳣ��Kֵ���� ��H2Ӧ�ӵ��ص�________ (��缫����)ͨ�룻����b�缫�ĵ缫��Ӧ����ʽΪ________��
��H2Ӧ�ӵ��ص�________ (��缫����)ͨ�룻����b�缫�ĵ缫��Ӧ����ʽΪ________��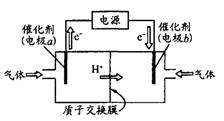
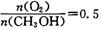 ʱ���Ʊ���Ӧ���������У�����һ����
ʱ���Ʊ���Ӧ���������У�����һ����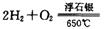
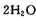 ������һ����____________ (д��ѧ����ʽ����
������һ����____________ (д��ѧ����ʽ����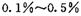 ��HCHO������ɱ����������ԭ����________��
��HCHO������ɱ����������ԭ����________��
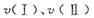 ��
��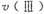 �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ�������� �ֱ�Ϊ
�ֱ�Ϊ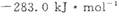 ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
 cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
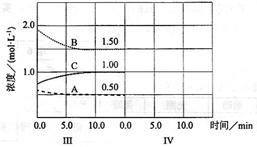
 ��һ�����ȷ�Ӧ����֪�÷�Ӧ����2molNH3ʱ���ų�92kJ������.
��һ�����ȷ�Ӧ����֪�÷�Ӧ����2molNH3ʱ���ų�92kJ������.