��Ŀ����
(12��)��ӦaA��g��+bB��g�� cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��

�ش����⣺
��1����Ӧ�Ļ�ѧ����ʽ�У�a��b��cΪ ��
��2��A��ƽ����Ӧ����vI��A����vII��A����vIII��A���Ӵ�С���д���Ϊ ��
(3) B��ƽ��ת����aI(B)��aII(B)��aIII(B)����С���� ����ֵ�� ��
��4���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ����� ����ȡ�Ĵ�ʩ�� ��
��5���Ƚϵ�II�η�Ӧ�¶ȣ�T1���͵�III�η�Ӧ�ٶȣ�T3���ĸߵͣ�T2 T3
��������������жϵ������� ��
��6���ﵽ������ƽ����������������һ�����ٶ�10min��ﵽ�µ�ƽ�⣬������ͼ�������߱�ʾIV����ϵ�и����ʵ�Ũ����ʱ��仯�����ƣ������ϱ�����A��B��C��.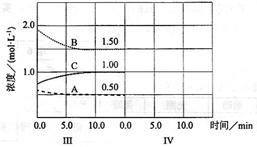
 cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
cC��g������H��0���ڵ��������½��С��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
�ش����⣺
��1����Ӧ�Ļ�ѧ����ʽ�У�a��b��cΪ ��
��2��A��ƽ����Ӧ����vI��A����vII��A����vIII��A���Ӵ�С���д���Ϊ ��
(3) B��ƽ��ת����aI(B)��aII(B)��aIII(B)����С���� ����ֵ�� ��
��4���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ����� ����ȡ�Ĵ�ʩ�� ��
��5���Ƚϵ�II�η�Ӧ�¶ȣ�T1���͵�III�η�Ӧ�ٶȣ�T3���ĸߵͣ�T2 T3
��������������жϵ������� ��
��6���ﵽ������ƽ����������������һ�����ٶ�10min��ﵽ�µ�ƽ�⣬������ͼ�������߱�ʾIV����ϵ�и����ʵ�Ũ����ʱ��仯�����ƣ������ϱ�����A��B��C��.

A


��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2C��g��������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����������ȷ���ǣ�������
2C��g��������2 s���룩����C��Ũ��Ϊ0.6 mol��L��1���������м���˵����������ȷ���ǣ�������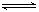 ?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����)
?mZ(g)����H����a kJ��mol��1(a>0)�����мס������ݻ�����ҹ̶����ܱ��������ڱ��ָ��¶Ⱥ㶨�������£����ܱ���������ͨ��2 mol X��1 mol Y���ﵽƽ��״̬ʱ���ų�����b kJ�����ܱ���������ͨ��1 mol X��0.5 mol Y���ﵽƽ��ʱ���ų�����c kJ����b>2c����a��b��m��ֵ���ϵ��ȷ����(����) CH3OH(g)��H2O(g) ��
CH3OH(g)��H2O(g) ��
 CH3OH (g) ��H����90.8 kJ��mol-1
CH3OH (g) ��H����90.8 kJ��mol-1 xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��
xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��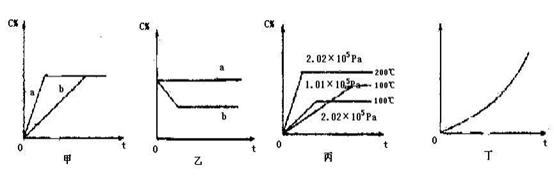
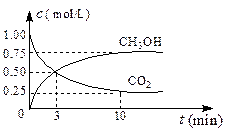
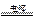 2H2����O2��
2H2����O2��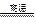 2H2����O2��
2H2����O2�� 2H2����O2��
2H2����O2��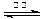 CO��3H2
CO��3H2 CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g) 
 ������5 min��ﵽƽ��״̬������ʱ���CH3OH������Ũ��Ϊ2 mol /L����
������5 min��ﵽƽ��״̬������ʱ���CH3OH������Ũ��Ϊ2 mol /L����