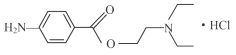
��Ŀ����
����Ŀ����֪�����Ȼ�ѧ����ʽ��
��H2(g)��1/2O2(g)��H2O(l)����H����285.8 kJ��mol��1
��H2(g)��1/2O2(g)��H2O(g)��H����241.8 kJ��mol��1
��C(s)��1/2O2(g)��CO(g)����H����110.5 kJ��mol��1
��C(s)��O2(g)��CO2(g)����H����393.5 kJ��mol��1��
�ش��������⣺
��1��������Ӧ�����ڷ��ȷ�Ӧ����____________��
��2��H2��ȼ����Ϊ____________ kJ��mol��1��C��ȼ����Ϊ____________ kJ��mol��1��
��3��ȼ��10 g H2����Һ̬ˮ���ų�������Ϊ____________ kJ��
��4��д��COȼ�յ��Ȼ�ѧ����ʽ__________________________________��
���𰸡��٢ڢۢ� 285.8 kJ /mol 393.5kJ /mol 1429 CO(g) +1/2O2 (g) ===CO2(g) ��H=��283kJ /mol
��������
(1)���ʵ�ȼ��Ϊ���ȷ�Ӧ�����ȷ�Ӧ����H��0��(2)ȼ������ָ��101kPʱ��1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������CԪ��ת��ΪCO2��HԪ��ת��ΪҺ̬ˮ���ݴ˷����ж���(3)������������ʵ�����Ȼ�����1mol����ȼ������Һ̬ˮʱ�ų�285.8kJ��������������(4)���ݸ�˹���ɿ�֪������-�ۿɵ�CO��ȼ�շ�Ӧ���ݴ˷������
(1)���ʵ�ȼ�վ�Ϊ���ȷ�Ӧ�����ȷ�Ӧ����H��0�����٢�Ϊ������ȼ�գ��ۢ�ΪC��ȼ�գ��ʢ٢ڢܾۢ�Ϊ���ȷ�Ӧ���ʴ�Ϊ���٢ڢۢ���
(2)ȼ������ָ��101kPʱ��1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������CԪ��ת��ΪCO2��HԪ��ת��ΪҺ̬ˮ���ʷ�Ӧ��Ϊ������ȼ���ȵ��Ȼ�ѧ����ʽ����������ȼ����Ϊ285.8kJmol-1����Ӧ��ΪC��ȼ���ȵ��Ȼ�ѧ����ʽ����C��ȼ����Ϊ393.5kJmol-1���ʴ�Ϊ��285.8kJmol-1��393.5kJmol-1��
(3)10g���������ʵ���Ϊ5mol����1mol����ȼ������Һ̬ˮʱ�ų�285.8,kJ����5mol����ȼ������Һ̬ˮʱ�ų�������Ϊ285.8kJmol-1��5mol=1429.0kJ���ʴ�Ϊ��1429.0��
(4)���ݸ�˹���ɿ�֪������-�ۿɵ�CO��ȼ�շ�ӦΪ��CO(g)+![]() O2(g)�TCO2(g) ��H=-283.0kJmol-1���ʴ�Ϊ��CO(g)+
O2(g)�TCO2(g) ��H=-283.0kJmol-1���ʴ�Ϊ��CO(g)+![]() O2(g)�TCO2(g) ��H=-283.0kJmol-1��
O2(g)�TCO2(g) ��H=-283.0kJmol-1��

����Ŀ��25 ��ʱ���������ʵĵ��볣�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO |
���볣�� | 1.7��10��5 | K1��4.3��10��7��K2��5.6��10��11 | 3.0��10��8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ______________��
��2��ͬŨ�ȵ�CH3COO����HCO3-��CO32-��ClO�����H����������ǿ������˳��Ϊ______��
��3��������0.1 mol��L��1��CH3COOH��Һ�ڼ�ˮϡ�����У����б���ʽ������һ����С����___________(�����)��һ���������________(�����)��һ��������________(�����)��
A��c(H��) B��c(H��)/c(CH3COOH) C��c(H��)��c(OH��)
D��c(OH��)/c(H��) E. c(CH3COO��)��c(H��)/c(CH3COOH)
������Һ�����¶ȣ�����5�ֱ���ʽ�������������____________(�����)��