��Ŀ����
����Ŀ��������ij��ȤС��Ժ�����ɳ�Ĵ��ν����ᴿʵ�������ͼ����ش��������⡣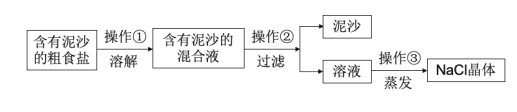
��1�����������õ��IJ���������__��
��2��������������������__��
��3����ʵ������NaCl�IJ���ƫ�ͣ�����ܵ�ԭ��__����ѡ���
A������ʱ��ֽ������
B������ʱû���ò���������
C���ܽ⺬��ɳ�Ĵ���ʱ������ˮ������
D�������Ȼ��ƾ���û�к�ɣ�����ˮ��
E�����˺����ֽ��ʪ�ģ�ֽ�ϵ�ˮ�ܽ���һЩ�Ȼ���
���𰸡���������©�����ձ� ���� BCE
��������
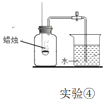
(1)�������ǹ��˲����������ǰѲ�����Һ��Ĺ�����Һ������һ�ַ��������˲�����װ��������̨���ձ�����������©������������ɣ����ڲ������������ձ�����������©����
(2)�������������������������������ǽ��裬��ֹ�ֲ��¶ȹ��ߣ����Һ��ɽ���
(3)A������ʱֽ�����𣬻ᵼ�����ʹ�õ��ľ����к�����ɳ�����ʣ����ᵼ�¾������Ȼ��Ƶ�����ƫ��ʹ���εIJ���ƫ�ߣ�
B������ʱû���ò��������裬�ᵼ�µõ��ľ��ε�����ƫС����ʹ���εIJ���ƫ�ͣ�
C���ܽ⺬��ɳ�Ĵ�ʳ��ʱ�������ˮ�����㣬�ᵼ�����ʹ�õ��ľ��ε�����ƫС����ʹ���εIJ���ƫ�ͣ�
D�����õ�NaCl����û�к�ɣ�����ˮ�֣����ᵼ�¾������Ȼ��Ƶ�����ƫ��ʹ���εIJ���ƫ�ߣ�
E�����˺���ֽ��ʪ�ģ�ֽ�ϵ�ˮ�ܽ���һЩ�Ȼ��ƣ��ᵼ�¾�������ƫС���Ӷ����¼�����ƫ�͡�

����Ŀ����ҵȼ��ú��ʯ�͵Ȼ�ʯȼ���ͷų�������������(NOx)��CO2��SO2�����壬������Ⱦ�������Է���������������̼����������ʵ����ɫ�������������á�
��.������
��֪H2��ȼ����Ϊ285.8kJ��mol��1
N2(g)��2O2(g)=2NO2(g) ��H����133kJ��mol��1
H2O(g)=H2O(l) ��H����44kJ��mol��1
���������£�H2��ԭNO2����ˮ���������������ʵ��Ȼ�ѧ����ʽΪ___��
��.��̼����1����2L�ܱ������м���2molCO2��6molH2�����ʵ��Ĵ��������£�������Ӧ��CO2(g)��3H2(g)![]() CH3OH(l)��H2O(l) ��H<0
CH3OH(l)��H2O(l) ��H<0
�ٸ÷�Ӧ�Է����е�������___(����¡������¡��������¶ȡ�)
������������˵���˷�Ӧ�ﵽƽ��״̬����___(����ĸ)��
a����������ƽ����Է����������ֲ��� b��CO2��H2������������ֲ���
c��CO2��H2��ת������� d�����������ܶȱ��ֲ���
e��1molCO2���ɵ�ͬʱ��3 molH��H������
��CO2��Ũ����ʱ��(0��t2)�仯��ͼ��ʾ����t2ʱ�������ݻ���Сһ����t3ʱ�ﵽƽ�⣬
t4ʱ�����¶ȣ�t5ʱ�ﵽƽ�⣬�뻭��t2��t6ʱ���CO2Ũ����ʱ��ı仯___��
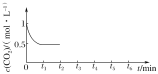
��2���ı��¶ȣ�ʹ��ӦCO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H<0�е��������ʶ�Ϊ��̬����ʼ�¶ȡ������ͬ(T1�桢2L�ܱ�����)����Ӧ�����в������ݼ��±���
CH3OH(g)��H2O(g) ��H<0�е��������ʶ�Ϊ��̬����ʼ�¶ȡ������ͬ(T1�桢2L�ܱ�����)����Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2(mol) | H2(mol) | CH3OH(mol) | H2O(mol) | |
��Ӧ�� ���º��� | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ�� ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�ٴﵽƽ��ʱ����Ӧ��Աȣ�ƽ��ʱCH3OH��Ũ��c(��)___c(��)(����>����<����������)��
�ڶԷ�Ӧ��ǰ10min�ڵ�ƽ����Ӧ����v(CH3OH)��___���������������������£���30 minʱֻ�ı��¶���T2�棬��ʱH2�����ʵ���Ϊ3.2mol����T1___(����>����<����������)T2����30minʱֻ���������ٳ���1 molCO2(g)��1molH2O(g)����ƽ��___(��������������������������)�ƶ���
��3�������˹�������ÿɽ�CO2ת��Ϊ���ᣬ��Ӧԭ��Ϊ2CO2��2H2O=2HCOOH��O2��װ����ͼ��ʾ��
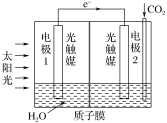
�ٵ缫2�ĵ缫��Ӧʽ��____��
���ڱ�״���£����缫2����11.2LCO2��Ӧ�������ϵ缫1��Һ������___(��������������������)___g��