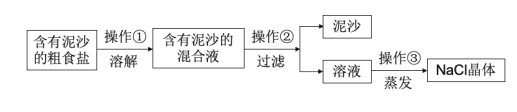
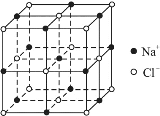
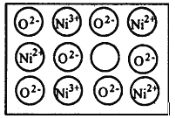
��Ŀ����
����Ŀ��Ԫ��X��Y��Z��W��M��Nԭ��������������X��M��W��N�ֱ�ͬ���壬��Ԫ��X��Y��Z��W�������������ڣ���������ԭ������֮��Ϊ22������������֮��Ϊ16����ش��������⣺
(1)д��Ԫ������ M_________________��N_____________________��
(2)д��M2W2�ĵ���ʽ________��Z2X4�ṹʽ__________________________��
(3)X��Z��W�γɵĻ������п��������ʵ���������ѧ����������________��
(4)ZX3��X2W���ܽ�Ⱥܴ��ԭ����Ҫ��__________________________________________��
(5)�����������������Z2X4��ȼ��X2W2����ȼ�������ﻷ������Ⱦ��д�����߷�Ӧ�Ļ�ѧ����ʽ_________��
(6)����X��W��M��N����Ԫ����ɵ����ֻ��������Ӧ���д̼�����ζ����ų�����������ͨ�����ᱵ��Һ�е����ӷ���ʽ________________________��
���𰸡��� �� ![]()
![]() ���Ӽ����ۼ� NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������� N2H4+ 2H2O2=N2+4H2O 3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+
���Ӽ����ۼ� NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������� N2H4+ 2H2O2=N2+4H2O 3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+
��������
Ԫ��X ��Y��Z��W��M��Nԭ��������������W��Nͬ���壬���ԭ��������֪��W���ڵڶ����ڡ� N���ڵ������ڣ���Ԫ��X��Y��Z��W�������������ڣ���Xֻ��ΪHԪ�أ� Y��Z���ڵڶ����ڣ� X��Y��Z��W��ԭ������֮��Ϊ22 ������������֮��Ϊ16����Y��Z��Wԭ������֮��Ϊ21������������֮��Ϊ15������֪YΪCԪ�ء�ZΪNԪ�ء�WΪOԪ�أ���NΪSԪ�أ�X��Mͬ���壬Mԭ����������0����MΪNa���ݴ˽��
(1)Ԫ�����ƣ�MΪ�ƣ�NΪ��
(2)M2W2ΪNa2O2�������ʽΪ![]() ��Z2X4ΪN2H4���ṹʽ
��Z2X4ΪN2H4���ṹʽ![]() ��
��
(3)X��Z��W�γɵĻ������п��������ʵ���ΪNH4NO3��������ѧ�������������Ӽ����ۼ���
(4)ZX3��X2W���ܽ�Ⱥܴ��ԭ����Ҫ��NH3��H2O�����м��ԣ���NH3���Ӻ�H2O����֮������γ�������������ܣ�
(5)�����������������N2H4��ȼ�ϡ�H2O2����ȼ��,���ﻷ������Ⱦ,��Ӧ���ɵ�����ˮ,���߷�Ӧ�ķ���ʽΪ:N2H4+ 2H2O2=N2+4H2O��
(6)����X��W��M��N����Ԫ����ɵ����ֻ�����NaHSO4��NaHSO3���Ӧ���д̼�����ζ����SO2�ų�����SO2����ͨ�����ᱵ��Һ�з�Ӧ�������ᱵ��һ����������Ӧ�����ӷ���ʽΪ3Ba2+ +3SO2+ 2NO3-+2H2O=3BaSO4��+2NO+4H+��

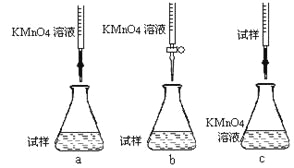
����Ŀ��ij�����Һ�п��ܴ������е��������±���ʾ��
������ | H����K����Al3����NH4+��Mg2�� |
������ | Cl����OH����CO32-��AlO2- |
Ϊ̽����ɷ֣�ijͬѧ��Na2O2���뵽���������Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ�ֱ���ͼ��ʾ��

(1)����Һ��һ�����е���������________________________________�����Ӧ���ʵ���Ũ��֮��Ϊ ____________����Һ��һ�������ڵ���������_______________________��
(2)д���������ٵ����ӷ���ʽ ________________________________________________��
����Ŀ���о�![]() ��
��![]() ��
��![]() �ȵĴ��������Ի�����������Ҫ���塣
�ȵĴ��������Ի�����������Ҫ���塣
��1����ѧ�������о����ô�������β���е�NO��COת���![]() ��
��![]() ���䷴ӦΪ��
���䷴ӦΪ��![]()
![]()
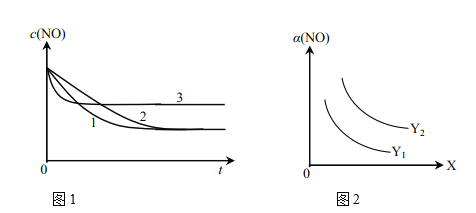
��Ϊ���о���������Ը÷�Ӧ��Ӱ�죬�����±�����ʵ�飬��ò�ͬʱ��NO��Ũ�ȣ�c����ʱ��仯��������ͼ1��ʾ��1��2��3������ʵ����������________������֪��ʹ�õ���������ʱ����������ȱ���������ѧ��Ӧ���ʡ���
ʵ���¶�NO��ʼŨ��O��ʼŨ�ȴ����ȱ��������������ţ��棩
ʵ�� ��� | �¶� ���棩 | NO��ʼŨ��
| CO��ʼŨ��
| �����ȱ����
| �������� ��g�� |
�� | 280 |
|
| 82 | 50 |
�� | 280 |
|
| 124 | 50 |
�� | 350 |
|
| 124 | 50 |
��ͼ2��ʾNO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�仯��ʾ��ͼ��X��ʾ����________��������________��Y��ʾ����________����Y1________Y2������>������<������
��2��һ���¶��£���![]() ��
��![]() �������1��2�����ܱ������з�����Ӧ
�������1��2�����ܱ������з�����Ӧ![]() ���ﵽƽ��ʱ
���ﵽƽ��ʱ![]() ���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��
���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��![]() ________��
________��
��3������ԭ��ط�Ӧ��ʵ��![]() ���������ܷ�ӦΪ
���������ܷ�ӦΪ ���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��
���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��