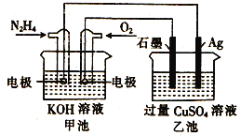
��Ŀ����
����Ŀ��������I(C11H12O3)���Ʊ�Һ�����ϵ��м���֮һ��������к���ȩ����������I������E��H��һ�������ºϳɣ�
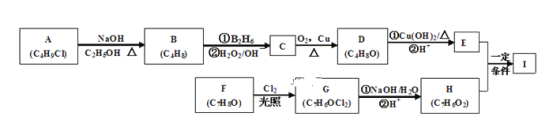
��֪���� A�ĺ˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣻
��R��CH��CH2![]() R��CH2CH2OH��
R��CH2CH2OH��
�ۻ�����F��ʹFeCl3��Һ������ɫ��Ӧ�������ϵ�һ�ȴ���ֻ�����֣�
��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
��1�� A�Ľṹ��ʽΪ��________________��B�Ļ�ѧ�����ǣ�___________________��
��2��C�Ľṹ��ʽΪ��_________________��F���������ŵ����ƣ�________________��
��3��A��B��G��H�ķ�Ӧ���ͷֱ�Ϊ��____________��___________��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ:B��C��_______________________��D��E����ٵķ���ʽ��_______________________________________��
��5��д����������������H�Ľṹ��ʽΪ��_____________________��
�ٲ���ʹFeCl3��Һ������ɫ��Ӧ�ķ����廯����
���ܷ���������Ӧ
�ۺ˴Ź�������������Ϊ��2:2:1:1
���𰸡�(CH3)3CCl 2-����ϩ (CH3)2CHCH2OH ���ǻ� ��ȥ��Ӧ ˮ�ⷴӦ����ȡ����Ӧ�� (CH3)2C=CH2 ![]() (CH3)2CHCH2OH (CH3)2CHCHO+2Cu(OH)2+NaOH
(CH3)2CHCH2OH (CH3)2CHCHO+2Cu(OH)2+NaOH![]() (CH3)2CHCOONa+Cu2O��+3H2O
(CH3)2CHCOONa+Cu2O��+3H2O 
��������
A�ķ���ʽΪC4H9Cl���˴Ź�������ֻ��һ�ֻ�ѧ�������⣬��AΪ(CH3)3CCl�����������Ƶ��Ҵ���Һ�����ȵ������·�����ȥ��Ӧ����B��BΪ(CH3)2C=CH2��B������Ϣ�еķ�Ӧ����CΪ(CH3)2CHCH2OH��C������������Ӧ����DΪ(CH3)2CHCHO��D����Cu(OH)2��Ӧ���ữ��õ�EΪ(CH3)2CHCOOH��F�ķ���ʽΪC7H8O����ʹFeCl3��Һ������ɫ��Ӧ˵����һ�����ǻ��������ϵ�һ�ȴ���ֻ�����֣�FӦ����2����ͬ�IJ����Ҵ��ڶ�λ����FΪ![]() ���������ڹ��������·���ȡ����Ӧ����GΪ
���������ڹ��������·���ȡ����Ӧ����GΪ![]() ��G����������ˮ��Һ�з���ˮ�⡢�ữ�õ�H��������ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����HΪ
��G����������ˮ��Һ�з���ˮ�⡢�ữ�õ�H��������ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����HΪ![]() ��H��E����������Ӧ����I�������к�����ȩ������������I
��H��E����������Ӧ����I�������к�����ȩ������������I �����������Ϸ������
�����������Ϸ������
��1���ɷ�����֪��A�ķ���ʽΪC4H9Cl���ṹ��ʽΪ(CH3)3CCl��BΪ(CH3)2C=CH2����ϵͳ����������Ϊ��2-����ϩ��
��2���ɷ�����֪C�Ľṹ��ʽΪ(CH3)2CHCH2OH��FΪ![]() ����������������Ϊ���ǻ���
����������������Ϊ���ǻ���
��3��A��B��±�������������Ƶ��Ҵ���Һ�����ȵ������·�����ȥ��Ӧ��G��H��±�������������Ƶ�ˮ��Һ�����ȵ������·���ˮ�ⷴӦ����ȡ����Ӧ�����ʴ�Ϊ����ȥ��Ӧ��ˮ�ⷴӦ����ȡ����Ӧ����
��4���ɷ�����֪B��C�ķ�Ӧ����ʽΪ�� (CH3)2C=CH2 ![]() (CH3)2CHCH2OH��D��E�����Ӧ�ڼ��Ի����з�Ӧ������ʽΪ(CH3)2CHCHO+2Cu(OH)2+NaOH
(CH3)2CHCH2OH��D��E�����Ӧ�ڼ��Ի����з�Ӧ������ʽΪ(CH3)2CHCHO+2Cu(OH)2+NaOH![]() (CH3)2CHCOONa+Cu2O��+3H2O��
(CH3)2CHCOONa+Cu2O��+3H2O��
��5��H�ķ���ʽΪC7H6O2���ٲ���ʹFeCl3��Һ������ɫ��Ӧ�ķ����廯���˵��������û���ǻ������ܷ���������Ӧ��˵������ȩ�������Ǽ���ij�����ͣ��ۺ˴Ź������������Ⱦ��Ƿ����е�Ч��ԭ�Ӹ���֮��Ϊ2��2��1��1���������������Ľṹ��ʽΪ�� ��
��

����Ŀ��ij�����Һ�п��ܴ������е��������±���ʾ��
������ | H����K����Al3����NH4+��Mg2�� |
������ | Cl����OH����CO32-��AlO2- |
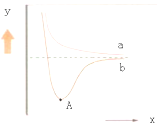
Ϊ̽����ɷ֣�ijͬѧ��Na2O2���뵽���������Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ�ֱ���ͼ��ʾ��

(1)����Һ��һ�����е���������________________________________�����Ӧ���ʵ���Ũ��֮��Ϊ ____________����Һ��һ�������ڵ���������_______________________��
(2)д���������ٵ����ӷ���ʽ ________________________________________________��