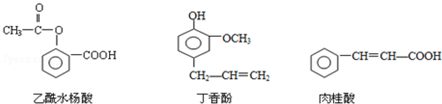
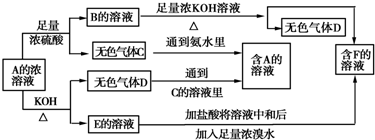
��Ŀ����
16����15.6g Na2O2��5.4g Alͬʱ����һ������ˮ�У���ַ�Ӧ��õ�200mL��Һ���������Һ�л���ͨ���״���µ�HCl����6.72 L������Ӧ��������Һ��������ֲ��䣬������˵����ȷ���ǣ�������| A�� | ��״���£���Ӧ�����еõ�7.84 L������ | |
| B�� | ���յõ�����Һ��c��Na+��=c��Cl-��+c��OH-�� | |
| C�� | ���յõ�7.8 g�ij��� | |
| D�� | ���յõ�����Һ��c��Na+��=1.5 mol•L-1 |
���� 15.6gNa2O2�����ʵ���Ϊ$\frac{15.6g}{78g/mol}$=0.2mol��5.4gAl�����ʵ���Ϊ$\frac{5.4g}{27g/mol}$=0.2mol�����ȷ�����Ӧ2Na2O2+2H2O�T4NaOH+O2��������NaOHΪ0.4mol���ٷ���2Al+2NaOH+2H2O�T2NaAlO2+3H2�����ɷ���ʽ��֪Al��ȫ��Ӧ��ʣ��NaOHΪ0.4mol-0.2mol=0.2mol������NaAlO2Ϊ0.2mol��ͨ���״���µ�HCl����6.72L�����ʵ���Ϊ$\frac{6.72L}{22.4L/mol}$=0.3mol�����ȷ�����ӦNaOH+HCl�TNaCl+H2O��ʣ��HClΪ0.3mol-0.2mol=0.1mol���ٷ�����ӦNaAlO2+HCl+H2O�TAl��OH��3��+NaCl���ɷ���ʽ��֪��NaAlO2��ʣ�࣬HCl��ȫ��Ӧ������Al��OH��3Ϊ0.1mol��������Һ������ΪNaAlO2��NaCl��
A�����ݹ���������ˮ��Ӧ�������������Ʒ�Ӧ���������������������������
B��������Һ������ΪNaAlO2��NaCl�����ݵ���غ��֪c��Na+��+c��H+��=c��Cl-��+c��AlO2-��+c��OH-����
C������m=nM�������ɵ�����������������
D������NaԪ���غ��֪��Ӧ����Һ��n��Na+��=2n��Na2O2�����ٸ���c=$\frac{n}{V}$���㣮
��� �⣺15.6gNa2O2�����ʵ���Ϊ$\frac{15.6g}{78g/mol}$=0.2mol��5.4gAl�����ʵ���Ϊ$\frac{5.4g}{27g/mol}$=0.2mol�����ȷ�����Ӧ2Na2O2+2H2O�T4NaOH+O2��������NaOHΪ0.4mol���ٷ���2Al+2NaOH+2H2O�T2NaAlO2+3H2�����ɷ���ʽ��֪Al��ȫ��Ӧ��ʣ��NaOHΪ0.4mol-0.2mol=0.2mol������NaAlO2Ϊ0.2mol��ͨ���״���µ�HCl����6.72L�����ʵ���Ϊ$\frac{6.72L}{22.4L/mol}$=0.3mol�����ȷ�����ӦNaOH+HCl�TNaCl+H2O��ʣ��HClΪ0.3mol-0.2mol=0.1mol���ٷ�����ӦNaAlO2+HCl+H2O�TAl��OH��3��+NaCl���ɷ���ʽ��֪��NaAlO2��ʣ�࣬HCl��ȫ��Ӧ������Al��OH��3Ϊ0.1mol��������Һ������ΪNaAlO2��NaCl��
A������������ˮ��Ӧ��������Ϊ0.2mol��$\frac{1}{2}$=0.1mol�������������Ʒ�Ӧ��������Ϊ0.2mol��$\frac{3}{2}$=0.3mol�����������������Ϊ��0.1mol+0.3mol����22.4L/mol=8.96L����A����
B����Ӧ����Һ�ijɷ���0.3molNaCl��0.1molNaAlO2���ɵ���غ��֪c��Na+��=c��Cl-��+c��OH-��+c��AlO2-��-c��H+������B����
C����������Al��OH��3Ϊ0.1mol������Ϊ0.1mol��78g/mol=7.8g����C��ȷ��
D������NaԪ���غ��֪����Ӧ����Һ��n��Na+��=2n��Na2O2��=2��0.2mol=0.4mol������Һ��c��Na+��=$\frac{0.4mol}{0.2L}$=2mol/L����D����
��ѡC��
���� ���⿼������ļ��㡢�ƵĻ������������ʼ�����������ʵȣ��Ѷ��еȣ����ݹ��������жϷ����ķ�Ӧ�ǽ���Ĺؼ���

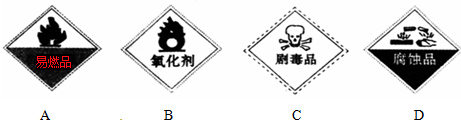
| A�� | ��ȩ | B�� | ���� | C�� | �״� | D�� | �ױ� |
| A�� | 1��17 | B�� | 15��8 | C�� | 14�� 6 | D�� | 12��9 |
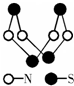
| A�� | �����ʵķ���ʽΪSN | |
| B�� | �����ʵķ�����ֻ�м��Լ���û�зǼ��Լ� | |
| C�� | �����ʷ�����N-S�����ܴܺ��侧���кܴ��Ӳ�� | |
| D�� | �������뻯����S2N2��Ϊͬ�������� |
| A�� | CH4��C2H4O2 | B�� | C2H6O��C2H4 | C�� | C3H6��C4H6 | D�� | C2H2��C6H6 |